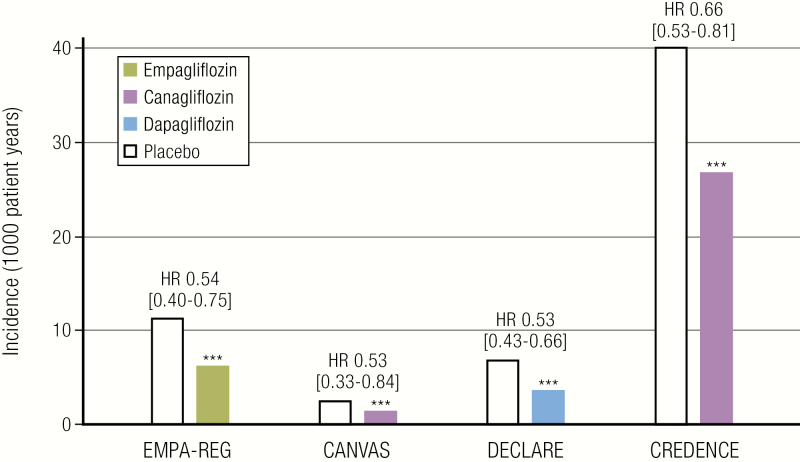
Figure 2.
Summary of renal composite outcomes with SGLT2 inhibition in EMPA-REG OUTCOME, CANVAS, DECLARE, and CREDENCE Trials. The renal composite outcome shown for EMPA-REG, CANVAS, and CREDENCE is incident or worsening nephropathy, defined as progression to macroalbuminuria; a doubling of the serum creatinine level; the initiation of renal-replacement therapy; or death from renal disease. For DECLARE, the renal composite outcome shown is sustained decline of at least 40% in estimated glomerular filtration rate to <60 mL/min/1.73 m2, end-stage renal disease, or death from renal causes. CANVAS, Canagliflozin Cardiovascular Assessment Study; CREDENCE, Evaluation of the Effects of Canagliflozin on Renal and Cardiovascular Outcomes in Participants with Diabetic Nephropathy; DECLARE, Dapagliflozin Effect on Cardiovascular Events; EMPA-REG, Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes; HR, hazard ratio.