Abstract
Neurodegenerative diseases (NDs) are a wide class of disorders of the central nervous system (CNS) with unknown etiology. Several factors were hypothesized to be involved in the pathogenesis of these diseases, including genetic and environmental factors. Many of these diseases show a sex prevalence and sex steroids were shown to have a role in the progression of specific forms of neurodegeneration. Estrogens were reported to be neuroprotective through their action on cognate nuclear and membrane receptors, while adverse effects of male hormones have been described on neuronal cells, although some data also suggest neuroprotective activities. The response of the CNS to sex steroids is a complex and integrated process that depends on (i) the type and amount of the cognate steroid receptor and (ii) the target cell type—either neurons, glia, or microglia. Moreover, the levels of sex steroids in the CNS fluctuate due to gonadal activities and to local metabolism and synthesis. Importantly, biochemical processes involved in the pathogenesis of NDs are increasingly being recognized as different between the two sexes and as influenced by sex steroids. The aim of this review is to present current state-of-the-art understanding on the potential role of sex steroids and their receptors on the onset and progression of major neurodegenerative disorders, namely, Alzheimer’s disease, Parkinson’s diseases, amyotrophic lateral sclerosis, and the peculiar motoneuron disease spinal and bulbar muscular atrophy, in which hormonal therapy is potentially useful as disease modifier.
Keywords: sex hormones, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, spinal and bulbar muscular atrophy
Graphical Abstract
Graphical Abstract.
ESSENTIAL POINTS.
Human neurodegenerative diseases are characterized by sex differences in term of onset and progression of disease, but current knowledge does not allow a precise definition of the sex-related factors intervening in these diseases.
Epidemiological and clinical studies linked the sex-specific synthesis of sex steroids to disease risk prevalence and incidence, but considering the hormonal pervasive effects on sexual differentiation and on brain development or functions, their sex-specific influence in neurodegeneration remains obscure.
The role played by sex steroids into functionally priming male and female brains also remains elusive, thus impairing our ability to understand the extent to which brain embryonal sex differentiation may be associated with the development of a sex-specific vulnerability to neuronal death in adulthood.
Present state-of-the-art knowledge does not allow us to definitely point to sex steroids as a direct or indirect components for protective or detrimental activities in these diseases.
The complexity of sex steroid physiological functions, the number of neural cells potentially involved, as well as epigenetic and environmental factors have impaired the understanding of the role of sex on neurodegeneration, so far.
We provide a wide and in-depth analysis of the role of sex in four common neurodegenerative diseases—Alzheimer’s disease, Parkinson’s diseases, amyotrophic lateral sclerosis, and spinal and bulbar muscular atrophy—because all show a sex-specific incidence and progression.
Our effort is aimed at facilitating the identification of all aspects that associate sex and disease manifestation at both preclinical and clinical level in these disorders to enable a progress in this field and underline potential ways where appropriate regulation of circulating hormones may provide benefits in these disorders where we suffer a unique lack of positive therapeutic intervention.
Neurodegenerative diseases (NDs) are devastating and largely fatal conditions that affect several million people in the world. NDs onset may occur during the entire life in humans, with juvenile forms appearing around birth (eg, some forms of spinal and muscular atrophy) and adult forms that may appear very late in life. Most NDs appear after the third decade of life, possibly correlating with the aging process. The causes leading to the massive neuronal death characteristic of NDs remain to be fully understood, thus developing strategies for their prevention, treatment, or even delayed progression is a major challenge for modern medicine. Although it is indisputable that the main symptoms of NDs reflect neuron-specific deficits, it is also conceivable that dysfunction in other brain cells could precede and facilitate neuronal loss. Nonneuronal cells in the central nervous system (CNS) include neuroglia (oligodendrocytes and astrocytes) and microglia, a class of cells maintaining brain homeostasis through an appropriate exchange of nutrients and protection against noxious stimuli (1). Oligodendrocytes enwrap axons with myelin sheaths providing a structural and local metabolic and homeostatic protection (2); microglia ensure immune protection, the control of synaptic connections and tissue repair (3, 4); astrocytes regulate the homeostasis of neurotransmission, metabolites, and reactive oxygen species (5). Compelling evidence shows that these cells are impaired in NDs (1, 6), as discussed in the appropriate sections of this review. Among the three major forms of ND, Alzheimer’s disease (AD) is more frequent in women than in men (ratio 2:1) (7), while Parkinson’s disease (PD) and amyotrophic lateral sclerosis (ALS) affect primarily men (ratios 1.5:1 and 2:1, respectively) (8, 9); peculiar is the case of spinal and bulbar muscular atrophy (SBMA), which affects men only (10). The molecular bases for this sex-related prevalence remain to be understood, although sex steroid hormones may be involved in disease pathogenesis. However, the decline of sex steroid synthesis occurring during aging or the changes due to pharmacological supplementation may be either a risk or protective factor as a function of the ND considered (11–13). Perhaps, the reason for these opposite results is that the connections between sex steroids and the manifestation of NDs are multifaceted as these hormones target all neuronal cells (neurons, glia, and/or the immune cells) and control brain sexual differentiation, a process that may be relevant for the degenerative processes in adults. Moreover, another element of complexity further impairing our ability to interpret the results of investigations is that, in addition to the gonads, sex steroids may be synthesized in the brain. Epidemiological evidence indicates a sexual prevalence and incidence in most forms of ND (Table 1).
Table 1.
The gender-related risk to develop neurodegenerative diseases
| Female | Male | |
| AD incidence | ||
| Animal models | - Higher Aβ accumulation (14); - Higher levels of hyperphosphorylated tau (14); - Poorer behavioral performance (15–19). | |
| Patients | - More severe β-amyloid accumulation (20, 21); - Faster cognitive decline (22, 23). | - Increased tau pathology (24). |
| PD incidence | ||
| Animal models | - Favorable performance using low neurotoxin doses, that mimic the early stages of the disease (25); - Higher sensibility to ubiquitin-proteasomal defects in PARK2 null mice (26). | - Significantly more robust reduction in DA levels in the striatum and loss of dopaminergic neurons in the SNpc (27–30). |
| Patients | - Older than males at symptom onset (31); - Present more often with a tremorigenic form and a slower progression of the disease (31). | - Increased risk due to the sexual dimorphism of the NSDA system (29). |
| ALS incidence | ||
| Animal models | - Outperform on batteries of behavioural tests measuring tremor, motor impairment, motor strength and coordination with rotarod and grip strength, as well as measuring tail elevation, footprint analysis, or motor activities (32, 33); - Earlier disease onset in mice co-expressing mutant chromogranin and SOD1 (34). | - SOD1 mutation decreased proliferative and differentiating potential of rat neural progenitor cells (35); - C-boutons enlargement (36); - The disease is significantly more aggressive; in fact, male ALS mice lose weight and show motor symptoms earlier than females (32, 37); - The disease progression is much shorter in males (32, 37). |
| Patients | - Older than males at the age of onset (38); - Prevalence of the bulbar form (38); - Significant vulnerability to develop cognitive dysfunctions related to ALS (39); - Positive association between longer reproductive time-span and susceptibility and survival of ALS (40). | - Prevalence of the forms with limb onset (38); - Higher CSF levels of SOD1 (41). |
| SBMA incidence | ||
| Animal models | - In tg mouse models, females are not affected (42, 43); - testosterone induces SBMA symptoms | |
| Patients | - Female carriers are asymptomatic or present mild clinical abnormalities (44, 45); - Women homozygous did not show any clinical symptom of ND (46). |
Abbreviations: Aβ, amyloid beta; ALS, amyotrophic lateral sclerosis; CSF, cerebrospinal fluid; DA, dopamine; ND, neurodegenerative disease; NSDA, nigrostriatal dopaminergic system; SBMA, spinal and bulbar muscular atrophy; SNpc, SN pars compacta; SOD1, superoxide dismutase 1.
Considering the reported beneficial versus deleterious effects of sex steroids, the aim of the present review is to recapitulate current knowledge on estrogen, progesterone, and androgen activities in neural cells and their potential influence on manifestation of NDs. The review will also analyze the impact of the relative contribution between circulating hormones, their local synthesis, and activation (Fig. 1), including their complex interplay in target cells (Fig. 2). Our anticipation is that a better understanding of this field of study may lead to novel therapeutic strategies very much in need for ND.
Figure 1.
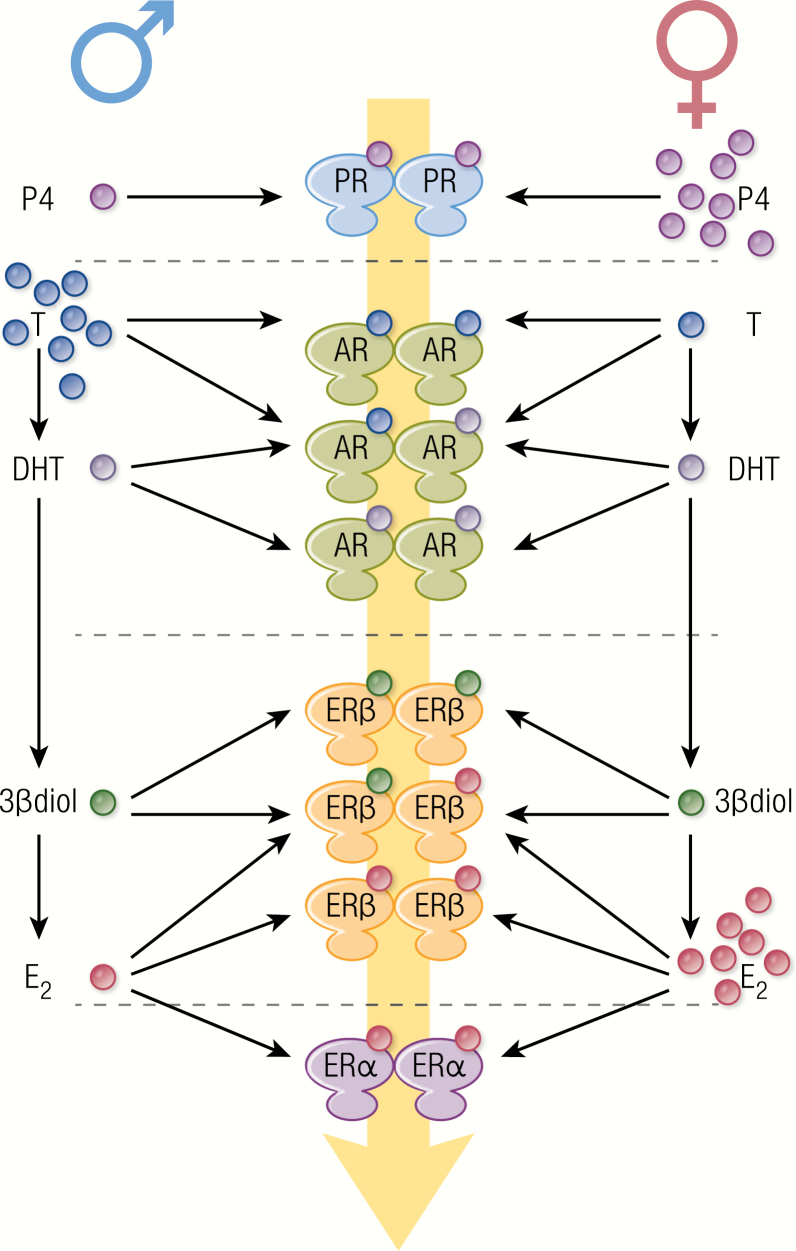
Biosynthetic pathways of neurosteroid formation. HMG-CoA is the precursor for cholesterol synthesis. HMG-CoA reductase catalyzes the production of mevalonic acid from HMG-CoA, with a reaction that is rate-limiting for cholesterol synthesis. Mevalonic acid is then converted via the the mevalonate pathway to lanosterol, which is, in turn, converted to cholesterol via either the Bloch pathway or the Kandutsch–Russell pathway. CYP11A1 (P450 side-chain cleavage, named P450scc in rodents), more expressed in females than in males, catalyzes the conversion of cholesterol to pregnenolone through the first rate-limiting step of de novo steroidogenesis. Further steroid conversions are catalyzed by 3β-HSD, CYP17 (P450c17), 5α-R, and CYP19 (aromatase). Full-line arrows indicate active steroidogenic biosynthetic pathways in the brain. Dashed line arrows represent undetermined steroidogenic biosynthetic pathways. Steroidogenic enzymes are represented by dashed line boxes. The color codes indicate their distribution in brain cells; cyan: neurons; green: microglia; orange: astrocytes.
Figure 2.
The complexity of sex hormone-receptor interactions in the nervous system. In neurons, 5α-R converts testosterone into DHT, which can be converted to 3β-diol by 3β-HSD, while aromatase converts testosterone to E2, giving rise to a variety of androgen metabolites, particularly in males. Blood-derived or locally produced sex hormones interact with specific homo or heterodimeric receptors, giving rise to a range of ligand-receptor complexes. Each hormone-receptor complex binds to gene promoters with specific preference and affinity, resulting in a combinatorial mechanism of transcriptional regulation by sex hormones within a cell. Furthermore, sex steroid receptors are expressed in neural and microglial cells in which they activate cell-specific genetic and metabolic programs. All these molecular and cellular mechanisms make up the response to the initial hormonal signal and participate in the sexual dimorphism of neurological functions.
The Influence of Sex on Neurodegenerative Diseases
Alzheimer’s disease: the pathogenesis
AD is the most common cause of dementia (47–49) and is a slowly progressive disease that begins well before the onset of clinical symptoms (50). With disease progression, memory loss and confusion become more severe (49), until impairments in basic physiological functions appear in the terminal stage of the disease. Hallmarks of AD include the accumulation of aggregates and deposits of misfolded proteins, like amyloid beta (Aβ) peptides, outside neurons, and neurofibrillary tangles (NFT), which contains abnormally phosphorylated microtubule-associated tau protein, inside neurons (50, 51). Increasing evidence points to a causal role of inflammation in disease onset (52).
Pathogenic mechanisms in familiar and sporadic AD.
Some AD cases (<1%) result from fully penetrant mutations of specific genes (Table 2), which associated with an onset risk before 65 years (69). Sporadic or late onset-form of AD (LOAD) manifests later in life and is linked to genetic factors (70) acting together with environmental risk factors (71) (Table 2). The most established genetic risk factor for LOAD is the ε4 allele of the apolipoprotein E gene (APOE), a major cholesterol carrier responsible for lipid transport in the brain (72, 73); APOEε4 accounts for differential risk to develop AD based on the gene dose (8.07 or 2.84 hazard ratio in homo- or heterozygosis, respectively) (56). Several other common genetic variants associated with LOAD highlighting the multifactorial origins of sporadic AD (Table 2). The blend of genetic, environmental, and epigenetic factors (74–76) leads to a progressive decrease in synaptic density and loss of integrity in neuronal networks, resulting into neuritic atrophy and neuronal death. Several causes are involved, such as amyloidoses, tauopathy, and impairment of lipid metabolism (77–80). In the case of APOEε4 carriers hypercholesterolemia is observed (~8% higher than baseline population) together with an increased susceptibility to develop amyloid deposition (81, 82). Other well-recognized etiopathogenetic elements are neuroinflammation (3, 52, 83, 84), impairment of autophagy (85), or lysosomal degradation (86), loss of Ca2+ homeostasis (87, 88), neuronal cycle control (89, 90), and metabolic dysregulation (91–93).
Table 2.
Genes/proteins involved in AD
| Gene symbol | Gene/protein name | Protein function | Reference | Sporadic (s) / familial (f) | Notes |
|---|---|---|---|---|---|
| APP | amyloid beta precursor protein | Precursor of beta amyloid (Aβ) | 53 | f | |
| ABCA7 | ATP binding cassette subfamily A member 7 | Regulates the homeostasis of phospholipids and cholesterol in the CNS | 54 | s | |
| ADAM10 | ADAM metallopeptidase domain 10 | The main α-secretase that cleaves APP in the nonamyloidogenic pathway | 55 | s | |
| ADAMTS4 | ADAM metallopeptidase with thrombospondin type 1 motif 4 | Facilitates Aβ4-x peptide generation | 55 | s | |
| ALPK2 | alpha kinase 2 | Protein serine/threonine kinase activity | 55 | s | |
| APH1B | aph-1 homolog B, gamma-secretase subunit | A functional component of the gamma-secretase complex | 55 | s | |
| APOE | apolipoprotein E | Lipid metabolism | 56 | s | The ε4 allele of APOE is the major genetic risk factor for LOAD |
| ATP5H | ATP synthase peripheral stalk subunit d | ATP biosynthetic process | 57 | s | |
| BIN1 | bridging integrator 1 | Involved in synaptic vesicle endocytosis | 58 | s | |
| CASS4 | Cas scaffold protein family member 4 | It may have a role in axonal transport | 59 | s | |
| CD2AP | CD2 associated protein | Regulates the actin cytoskeleton | 54 | s | |
| CD33 | CD33 molecule | It may have a role in cell-to-cell adhesion | 54 | s | Expressed on cells of myeloid lineage |
| CELF1 | CUGBP Elav-like family member 1 | Involved in pre-mRNA alternative splicing, mRNA translation and stability. | 59 | s | |
| CLNK | cytokine dependent hematopoietic cell linker | Plays a role in the regulation of immunoreceptor signaling | 55 | s | Expressed on cells of myeloid lineage |
| CLU | clusterin | A Golgi chaperone that facilitates the folding of secreted proteins | 60 | s | |
| CNTNAP2 | contactin associated protein like 2 | It may play a role in the local differentiation of the axon | 55 | s | |
| CR1 | complement C3b/C4b receptor 1 (Knops blood group) | Act as a negative regulator of complement cascade, mediating phagocytosis | 61 | s | |
| DRB1 | RNA binding motif protein 45 | Binding to poly(C) RNA | 59 | s | |
| EPHA1 | EPH receptor A1 | Regulates cell proliferation, may play a role in apoptosis | 54 | s | |
| FERMT2 | fermitin family member 2 | Regulates the activation of integrins | 59 | s | |
| HESX1 | HESX homeobox 1 | Involved in forebrain development | 55 | s | |
| INPP5D | inositol polyphosphate-5-phosphatase D | Regulation of cellular activation | 59 | s | |
| KAT8 | lysine acetyltransferase 8 | Selectively inhibits antiviral immunity | 55 | s | |
| MAPT | microtubule associated protein tau | Encodes for tau, the predominant component of neurofibrillary tangles | 62 | f | Unclear Pathogenicity for AD |
| MEF2C | myocyte enhancer factor 2C | Involved in neurogenesis and in the development of cortical architecture | 59 | s | |
| MS4A | membrane-spanning 4A | Participates in the regulation of calcium signaling | 54 | s | |
| NME8 | NME/NM23 family member 8 | Nucleoside diphosphate kinase activity | 59 | s | |
| PICALM | phosphatidylinositol binding clathrin assembly protein | Involved in cellular trafficking and regulation of endocytosis | 60 | s | |
| PLD3 | phospholipase D family member 3 | Involved in APP processing | 63 | s | |
| PSEN1 | presenilin 1 | Part of the γ-secretase complex, regulating APP processing | 64 | f | |
| PSEN2 | presenilin 2 | Part of the γ-secretase complex, regulating APP processing | 65 | f | |
| PTK2B | protein tyrosine kinase 2 beta | Enhances signals that regulate neuronal activity | 59 | s | |
| RIN3 | Ras and Rab interactor 3 | Involved in the early endocytic pathway | 59 | s | |
| SORL1 | sortilin related receptor 1 | A neuronal apolipoprotein E receptor | 59 | s | |
| TREM2 | triggering receptor expressed on myeloid cells 2 | Phagocytosis, migration, activation | 66 | s | Exclusively expressed on cells within the myeloid lineage. SNPs increase disease risk by 2- to 4-fold. |
| TRIP4 | thyroid hormone receptor interactor 4 | Paly a role in the estrogen receptor and NF-κB transactivation | 67 | s | |
| UNC5C | unc-5 netrin receptor C | Direct axon extension and cell migration during neural development | 68 | s | |
| ZCWPW1 | zinc finger CW-type and PWWP domain containing 1 | A histone modification reader involved in epigenetic regulation | 59 | s |
Abbreviations: AD, Alzheimer’s disease; APP, amyloid precursor protein, Aβ, amyloid beta, ATP, adenosine triphosphate; CNS, central nervous system; APOE, apolipoprotein E, mRNA, messenger RNA; NF-κB, nuclear factor kappa B; RNA, ribonucleic acid.
Nonneuronal cells in AD.
Neuroinflammation in AD is due to a persistent activation of microglia associated with massive release of molecules toxic to neurons and oligodendrocytes (eg, glutamate, free radicals, and tumor necrosis factor alpha [TNFα] (52)). Indeed, in early AD stages, microglia is neuroprotective by clearing Aβ deposits and by internalizing protofibrillar and fibrillar forms of Aβ peptide via endocytosis or macropinocytosis (94–97). This activity decreases during disease progression, when Aβ receptor expression (such as SRA, CD36, RAGE) is reduced and the degradation of the engulfed amyloid fibrils is impaired. In turn, the formation of amyloid deposits within microglia sustains the production of proinflammatory cytokines (interleukin 1 beta [IL-1β], interleukin 6, and TNFα) detrimental to neurons (83, 98). Moreover, with aging, microglia becomes dystrophic, showing morphological characteristics distinct from young, healthy cells (3, 99); this process is further aggravated in pathologies involving systemic inflammation (eg, obesity, diabetes mellitus) (83, 100, 101).
Brain aging is often associated with a loss of astrocyte functions (6), prior to AD plaque or tangle formation (102). Myelin breakdown is induced by neuroinflammation and oxidative stress, and it also occurs at early stages of AD, before AD plaque or tangle formation (2), but its relevance for AD pathogenesis and the reasons why oligodendrocytes are unable to repair the initial myelin breakdown remain to be elucidated.
Sex differences in AD.
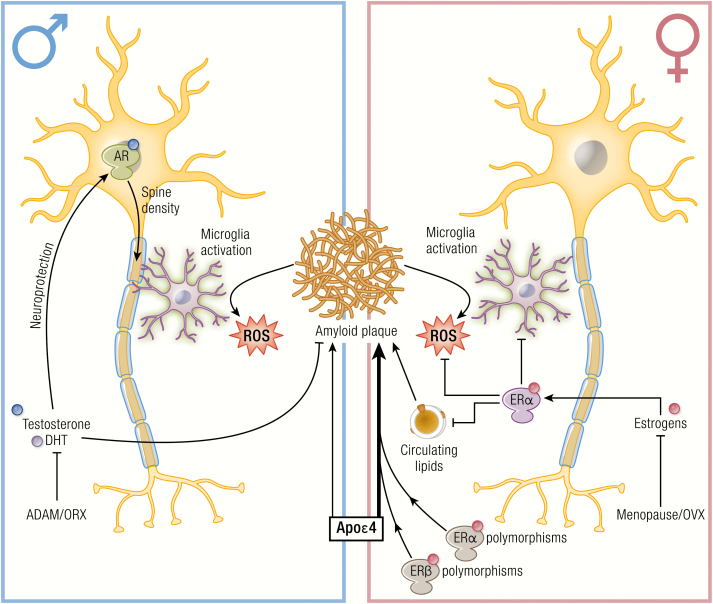
Besides age and the genetic risk factors, sex also strongly influences the risk of AD. In fact, it is estimated that almost two-thirds of Americans with AD are women (48), while among people aged 71 and older, 16% of women have AD compared with 11% of men (103, 104). Also the cognitive decline is sexually dimorphic and is faster in females (22, 23) with AD, but not in mild cognitive impairment (MCI) or other forms of dementia (105, 106). Multimodality brain imaging indicates that in females biomarkers of the preclinical phase of AD, including failures in cerebral glucose metabolism and a decrease in neuron mitochondrial function, appear early and overlap the endocrine transition of perimenopause (107). Neuropathological studies evidenced a more severe β-amyloid accumulation in women (20, 21) and increased levels of tau pathology in men (24). These pathological hallmarks are reflected in most AD animal models (Tg2576, amyloid precursor protein [APP]swe/PSEN1E9, APP23, APPswexPS1, and 3xTg-AD). Notably, a poorer behavioral performance and a greater increase of Aβ accumulation and hyperphosphorylated tau levels are observed in females than age-matched males (14–19). Several biological and social reasons were proposed to explain the AD prevalence in women (7), including the fact that women live longer than men, and older age is the greatest risk factor for AD (108). It has been suggested that men surviving to older ages may have a healthier cardiovascular risk profile, thus a lower risk for dementia than women of the same age (109); however the fact that the higher AD incidence in female is present in all age-matched groups (from 60–64 until 95 years of age) does not support this hypothesis. The role of sex hormones is still to be clarified, since mixed results were reported on the use of hormone replacement therapy (HRT) used to counteract the development and progression of AD. A strong association between APOε4 genotype with sporadic AD in women has been reported (110, 111). Indeed, women carrying both the homo- and heterozygous Apoε4 isoform have a higher rate of conversion from healthy aging to MCI and from MCI to AD. Conversely, Apoε4 variant in men has marginal effects when in homozygosity and null in heterozygosity (110, 112). Memory tests done in individuals with ε3/ε3, ε3/ε4, and ε4/ε4 APOE genotypes showed a significant gene–dosage dependent effect of the ε4 allele on performance, and male ε4 carriers at midlife showed a significant behavioral advantage in short-term memory task as compared with women (113).
Parkinson’s disease: the pathogenesis
PD is a progressive ND affecting 1% to 2% individuals over 65 years of age. It is characterized by motor symptoms that include tremor at rest, rigidity, and bradykinesia, as well as nonmotor symptoms (cognitive impairment and mood, olfaction and autonomic dysfunctions). Motor symptoms are due to the selective degeneration of dopaminergic neurons located in the substantia nigra (SN) and innervating the striatum, forming the nigrostriatal dopaminergic (NSDA) system. Dopaminergic neurons have a high oxidative status at baseline, possibly because of the natural propensity of dopamine (DA) to oxidation. Antioxidant and detoxification systems are thus mainly devoted to control DA metabolites in dopaminergic neurons; this makes the NSDA system more susceptible and vulnerable to even mild forms of oxidative stress and energy dysmetabolism, which instead spare other neuronal cell types (114, 115). PD is characterized by Lewy’s bodies, which are intraneuronal aggregates mainly consisting of α-synuclein (SNCA) protein (116) in the central and enteric nervous systems. This suggested a gut-to-brain spreading of aggregated SNCA possibly due to exposure to environmental toxins (117, 118).
Common pathogenic mechanisms in genetic and idiopathic PD.
Familial PD (fPD) is rare and generally monogenic, while most PD are sporadic forms (85%–90%) originating from both genetic causes and environmental factors (eg, pesticides and metals) (119, 120). Some causative genes and genetic predisposition factors have been identified (Table 3) and suggested key molecular pathways as major elements in PD pathogenesis: (i) protein aggregation, since SNCA gene mutations render the protein (and its DA-induced oxidized forms) prone to oligomerize in neurons impairing protein trafficking and degradation (129–131); (ii) mitochondrial defects and oxidative stress, since mutations in genes encoding leucine-rich repeat serine/threonine kinase 2 (LRRK2), DJ-1, PTEN-induced putative kinase 1 (PINK1), and Parkin produce mutated proteins causing respiratory chain dysfunctions and oxidative stress (115, 132); (iii) protein and mitochondria quality systems, like autophagy and the lysosomal pathways, since mutations have been found in the glucocerebrosidase gene (GBA) (133–138); and (iv) neuroinflammation, since polymorphisms in proinflammatory genes potentiate the oxidative microenvironment induced by DA, its metabolites, and oligomeric SNCA (139–145).
Table 3.
Genes/proteins involved in PD
| Gene symbol | Gene/protein name | Protein function | Reference | Sporadic (s) / familial (f) | Notes |
|---|---|---|---|---|---|
| ATP13A2 | ATPase cation transporter 13A2 | lysosomal transmembrane protein associated with inorganic cations transport | 121 | f | Mutations produce truncated inactive proteins and cause an atypical form of PD (Kufor-Rakeb syndrome), with juvenile onset and rapid progression. Recessive inhheritance. |
| DJ-1 | DJ-1 | Molecular chaperone which acts as cellular sensor of oxidative stress and protecits mitochondria | 122 | f | Earky onset and slow disease progression. Recessive inhheritance. |
| GBA1 | Beta-glucosylceramidase—glucocerebrosidase | lysosomal enzyme involved in glycolipid metabolism | 123,124 | s | Heterozygous mutations cause accumulation of glucocerebroside in different organs and are considered the greatest genetic risk factor for PD also associated with reduced age of onset. |
| LRRK2 | Leucine-rich repeat kinase 2 | neurite and synapse formation, membrane trafficking, autophagy | 125 | s/f | Missense mutations result in amino acid substitutions that increase protein activity. Mutations in the LRKK2 gene are the most common cause of inheritable and sporadic PD, with mid-to-late onset and slow progression. Dominant inheritance. |
| PINK1 (PARK6) | PTEN-induced putative kinase | serine-threonine mitochondrial kinase involved in mitochondrial function together with parkin | 126 | f | Truncating, missense and nonsense point and frameshift mutations result in dysfunctional protein; early onset. Recessive inhheritance. |
| PRKN | Parkin | E3 ubiquitin ligase regulating mitochondrial function and protein quality control system | 127 | s/f | It is the most common cause of autosomal recessive PD, with early onset (less than 40 years old) and slow progression. Recessive inheritance |
| SNCA | α-synuclein | synaptic function and neurotransmission | 128 | s (rarely)/f | Amino acid substitutions, due to missense mutations or increased protein expression, due to gene locus multiplications, render α-syn prone to aggregation. Dominant inheritance. |
Abbreviation: PD, Parkinson’s disease.
Animal models of PD are widely used to study disease pathogenesis, particularly those obtained by pharmacological treatments. Injection of the DA analogues, 6-hydroxydopamine (6-OHDA) and metamphetamine (ME); the DA transporter substrates, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP); or the pesticide rotenone fully reflect the biological defects of fPD (146) by inducing DA accumulation and production of reactive and oxidative species or by inhibiting mitochondrial activity and inducing oxidative stress and energy failure. This points to oxidative stress as a major driver of dopaminergic neuron degeneration, sustained by an interconnected network of genetic and biochemical alterations and inflammation. Oxidative stress induces chemical modification of deoxyribonucleic acid (DNA), proteins, and lipids in PD brains and promotes protein aggregation engulfing the detoxification systems in dopaminergic neurons (147–151).
Nonneuronal cells involved in PD.
Besides neuron-specific defects, nonneuronal cells may be involved in PD pathogenesis. Microglia activation is present in PD patients and animal models, even before neuronal loss or the activation of other glial cells. Microglia may be detrimental to PD by secreting neurotoxic inflammatory mediators and by a limited efficiency in removing misfolded proteins by phagocytosis. Indeed, pharmacological inhibition of microglia is neuroprotective (152–163). Interestingly, microglia activation in the whole brain leads to dopaminergic neuron death only in the NSDA system, suggesting a key role for microglia and inflammation in PD motor defects (164–169). Astrogliosis is detected in specific brain regions of PD patients and animal models of the disease (170), and astrocyte contribution to PD pathogenesis is dual. On one side, activated astrocytes release inflammatory and oxidative stress mediators and sustain iron-induced neurotoxicity; on the other side, astrocytes display induction of autophagy and produce neuron survival factors (171–173). Importantly, many genes involved in PD are also expressed by astrocytes, suggesting that pathogenic signals may also originate from these cells (174). A sexually dimorphic reactivity of astrocytes has been reported in response to MPTP or lipopolysaccharide (LPS), giving rise to specific patterns of adenosine triphosphate (ATP)/reactive oxygen species (ROS) and inflammatory cytokines production in astrocytes generated from male or female brains (170, 175), supporting the hypothesis that they may contribute to sex differences in neuroinflammatory diseases (176).
Sex differences in PD.
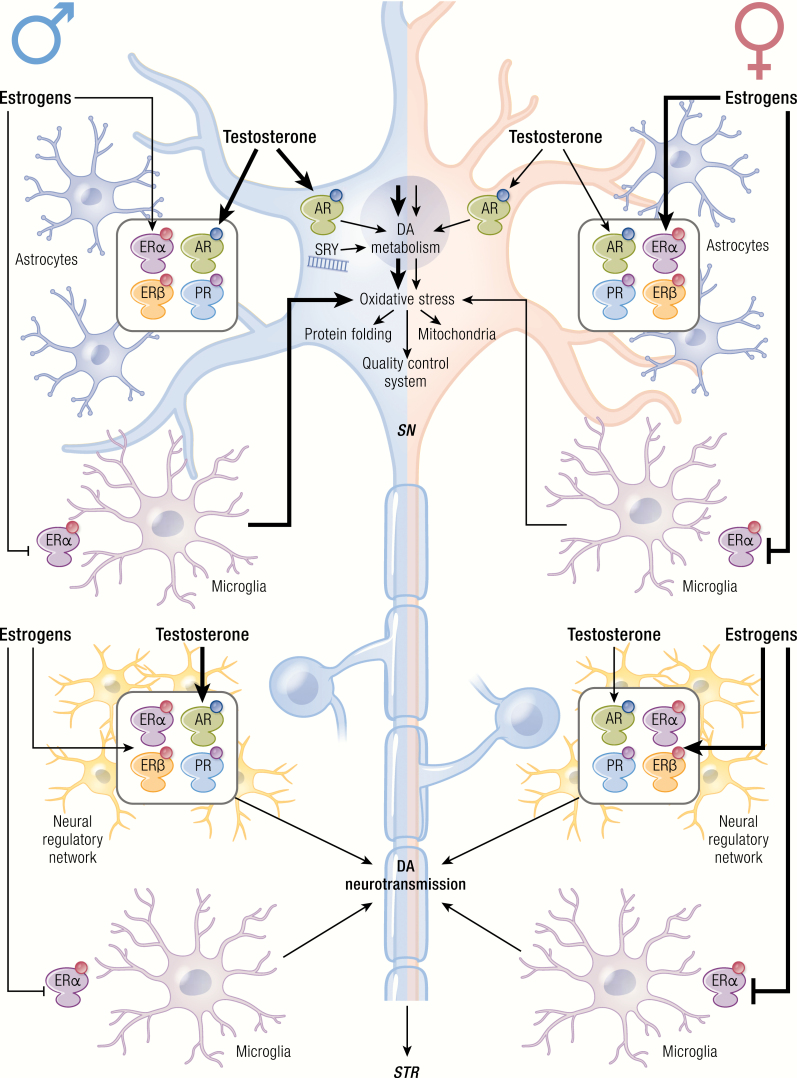
Together with aging, male sex is the strongest risk factor for PD. The risk to develop PD is 1.5-fold greater in men than in women at all ages (31, 177) (Table 1). The phenotypic characteristics of the disease are also sexually dimorphic, since women tend to be older than men at symptom onset and more often present a tremorigenic form and a slower disease progression (31). Genomic profiling of the SN pars compacta (SNpc) neurons from healthy donors and PD patients further substantiated the different role that biological traits of men and women may have in disease etiology, symptoms, and response to therapy (178). The difference in PD incidence among the two sexes may arise from substantial distinctions in healthy adult men and women in the composition and reactivity of the NSDA system, and this is a peculiar feature of PD among other NDs. As compared to women, the SNpc in men shows (i) a higher number of dopaminergic neurons with increased expression of SNCA and PINK1; (ii) more robust DA release induced by stimuli, such as psychomotor stimulants; and (iii) increased vulnerability to selective drugs in terms of addiction and toxicity. Sexual differences are also observed in regulatory systems controlling NSDA plasticity, a complex network made of interneurons and glia, microglia, and input circuitries from other brain areas that is sexually distinct in terms of cell composition, function, and adaptation to signals. Male-specific genetic determinants and not organizational effects of sex steroid hormones appear to define this sexual dimorphism in NSDA circuitry. Importantly, the Y chromosome-specific gene SRY (sex-determining region Y) is expressed in NSDA neurons of males, and its activity is associated with a positive regulation of neuron number, DA synthesis, and metabolism (179, 180). It is thus proposed that intrinsic biological properties related to SRY gene expression predispose men to disorders associated with dopaminergic abnormalities in PD or schizophrenia.
Sex-related differences are also observed in the SNpc of rodents (26, 27) and in the susceptibility and progression of PD models. The loss of dopaminergic neurons in the SNpc and the reduction in DA levels in the striatum are more robust in males, whereas the female sex is neuroprotective when low doses of neurotoxins are used to evidence early stages of PD (27, 28, 30). Genetic models are only recently being studied in terms of sexual differences; neurodegeneration is increased in PARK2-null female mice, as compared with males, suggesting a higher sensibility of females to ubiquitin-proteasomal defects (26, 146). Neuronal primary cultures from females survive longer and adapt to starvation through distinct metabolic programs, while autophagy is associated with cell death in male cells (181).
Oxidative stress and mitochondrial functions are also sexually dimorphic in specific brain areas; females have increased antioxidant defenses and respiratory chain activity compared to males, thus with lower mitochondrial ROS production and oxidative damage as a consequence of the higher expression of mitochondrial proteins and antioxidant enzymes (eg, paraoxynase-2 or thioredoxin) (182–185). Male mesencephalic neurons exposed to 6-OHDA have reduced expression of selected mitochondrial proteins, lower ATP levels and higher ROS levels compared to female neurons (186). Moreover, females show a higher stress adaptation than males mediated by the expression of stress response factors and the differential mitochondrial usage of energy sources (181, 187).
Sexual differences in neuroinflammation have been scarcely analyzed in PD patients and in animal models of the disease. Using MPTP, the increase in TNFα, IL-1β and interferon gamma (IFNγ) is faster and correlates with earlier and greater decrease of tyrosine hydroxylase (TH) neurons in male than female mice striatum (188).
Amyotrophic lateral sclerosis: the pathogenesis
Amyotrophic lateral sclerosis has an incidence of 1–2:100 000 and is primarily associated with functional alterations of the upper and lower motoneurons (placed in cerebral motor cortex or brainstem and in the ventral horns of the spinal cord or in the cranial nerves, respectively) and their target muscle cells. Neurons in the frontotemporal regions of the brain are seldomly involved (189), and ALS may manifest as pure motor form or associated with frontotemporal dementia (ALS-FTD). Age of onset and progression rate are highly variable (onset generally occurs around 50–60 years of age; juvenile forms are rare) (189).
Sporadic versus familial forms of ALS.
ALS occurs in two, clinically indistinguishable sporadic (sALS) and familial (fALS) forms. fALS occurs only in 10% to 15% of patients (see Table 4 for details). Several genes are associated with fALS (189); the first identified is present in 20% of fALS and encodes the antioxidant enzyme superoxide dismutase 1 (SOD1), a free radical scavenger enzyme ubiquitously expressed (232). More recently described gene mutations include the genes encoding TAR DNA-binding protein 43 (TDP-43), ALS-linked fused in sarcoma/translocated in liposarcoma (FUS/TLS), optineurin, and others (a list of gene mutations linked to ALS is reported in Table 4). Generally, the genetic alteration is associated with a gain in toxic function due to altered protein conformation (233) (misfolding) and accumulation as protein aggregates poorly cleared by motoneurons. Alternatively, the mutation affects essential biological functions by interfering with ribonucleic acid (RNA) functions, axonal transport, and mitochondrial and/or proteasome activities (234). Notably, the proteins found mutated in fALS may show aberrant behavior also in their wild type (wt) form in sALS (eg, oxidized wtSOD1, cleaved C-terminus of wtTDP-43, etc.) (235–238), suggesting the existence of common pathological mechanisms in fALS and sALS. These proteins may thus have a natural propensity to misfold and aggregate, forming insoluble inclusions.
Table 4.
Genes/proteins involved in motoneuron diseases (ALS and SBMA)
| Gene symbol | Gene/protein name | Protein function | Reference | Sporadic (s) / familial (f) | Notes |
|---|---|---|---|---|---|
| ALS | |||||
| ALS2 | Alsin Rho guanine nucleotide exchange factor ALS2 | Alsin | Rho Guanine Nucleotide Exchange Factor | 190 | f | |
| ANG | Angiogenin | Actin-binding protein; ribonuclease | 191 | s/f | |
| ANXA11 | Annexin A11 | Vesicle trafficking, apoptosis, exocytosis, and cytokinesis | 192 | s/f | |
| ATXN2 | Ataxin 2 | Endocytosis/RNA metabolism | 193 | s/f | CAG repeat sequence longer than 35 CAG causes Spino Cerebellar Ataxia (SCA)-2; shorter repeats are linked to ALS. |
| C9orf72 | C9orf72-SMCR8 complex subunit | Guanine nucleotide exchange C9orf72 | Guanine nucleotide exchange factor—involved in autophagy | 194,195 | s/f | Abnormal GGGGCC (G4C2) expansion are abnormally translated (sense and anti-sense mRNAs) by RAN-translation producing five dipeptide-repeat (DPR) proteins |
| CCNF | Cyclin F | Catalyzes ubiquitin transfer to substrates for UPS degradation | 196 | s/f | |
| CFAP410 | Cilia and flagella associated protein 410 | Regulation of cell morphology and cytoskeletal organization | 197 | s/f | Also: chromosome 21 open reading frame 2 (C21orf2) |
| CHCHD10 | Coiled-coil-helix-coiled-coil-helix domain-containing protein 10 | Mitochondrial protein | 198 | s/f | |
| CHMP2B | Charged multivesicular body protein 2B | Component of the endosomal sorting required for transport complex III (ESCRT-III); involved in sorting of endosomal cargo proteins. | 199 | f | May also be involved in FTD |
| DAO | D-amino acid oxidase | Regulates the level of the neuromodulator D-serine in the brain | 200 | f | May also be involved in FTD |
| DCTN1 | Dynactin subunit 1 | Component of dynein motor complex | 201 | s/f | May also be involved in FTD |
| ELP3 | Elongator acetyltransferase complex subunit 3 | Elongator complex protein 3 | RNA polymerase II component | 202 | s | |
| ERBB4 | Erb-b2 receptor tyrosine kinase 4 | Member of the epidermal growth factor (EGF) receptor tyrosine kinases | 203 | s/f | |
| EWSR1 | EWS RNA binding protein 1 | RNA-binding protein EWS | RNA/DNA binding protein | 204 | s | Previous name: Ewing sarcoma breakpoint region 1 |
| FIG4 | FIG4 phosphoinositide 5-phosphatase | Polyphosphoinositide phosphatase | Regulates synthesis and turnover of phosphatidylinositol 3,5-bisphosphate | 205 | s/f | |
| FUS | FUS RNA binding protein | RNA-binding protein | 206,207 | s/f | |
| GLE1 | GLE1 RNA export mediator | Nucleoporin GLE1 | Required for the export of mRNAs from the nucleus to the cytoplasm | 208 | s/f | |
| HNRNPA1 | Heterogeneous nuclear ribonucleoprotein A1 | RNA-binding protein | 209 | s/f | |
| HNRNPA2/B1 | Heterogeneous nuclear ribonucleoprotein A2/B1 | RNA-binding protein | 209 | s | Mutations in HNRNPA2B1 associated with ALS are extremely rare |
| KIF5A | Kinesin family member 5A | Kinesin heavy chain isoform 5A | Microtubule-based motor protein | 210 | s | |
| MATR3 | Matrin 3 | RNA-binding protein | 211 | s/f | May also be involved in FTD |
| NEFH | Neurofilament heavy | Neurofilament heavy polypeptide | Cytoskeletal component | 212 | s/f | |
| NEK1 | NIMA Related Kinase 1 | Serine/threonine-protein kinase Nek1 | Cytoskeletal dynamics | 213 | s/f | |
| OPTN | Optineurin | Autophagy adaptor | 214 | s/f | |
| PFN1 | Profilin 1 | Actin-binding protein | 215 | s/f | |
| SETX | Senataxin; Probable helicase senataxin | DNA/RNA helicase | 216 | s | |
| SIGMAR1 | Sigma non-opioid intracellular receptor 1 | Lipid transport from the endoplasmic reticulum | 217 | f | May also be involved in FTD |
| SOD1 | Superoxide dismutase 1 | Superoxide dismutase | 218 | s/f | First ALS identified gene |
| SPG11 | SPG11 vesicle trafficking associated, spatacsin | Spatacsin | Maintenance of cytoskeleton stability/regulation of synaptic vesicle transport | 219 | f | |
| SQSTM1 | Sequestosome 1 | Autophagy adaptor | 220 | s/f | May also be involved in FTD |
| TAF15 | TATA-box binding protein associated factor 15 | RNA-binding protein | 221 | s/f | |
| TARDBP | TAR DNA Binding Protein | TAR DNA-binding protein 43 | RNA-binding protein | 222,223 | s/f | TDP-43 and its C-terminal fragments (TDP-35 and TDP-25) are component of pathological inclusions found in almost all ALS patients (not in mutant SOD1s). May also be involved in FTD |
| TBK1 | TANK binding kinase 1 | Serine/threonine-protein kinase TBK1 | Innate immune response, autophagy, inflammation and cell proliferation | 224 | s/f | |
| TIA1 | TIA1 cytotoxic granule associated RNA binding protein | Nucleolysin TIA-1 isoform p40 | RNA-binding protein | 225 | s/f | |
| TUBA4A | Tubulin alpha 4a | Microtubules subunit | 226,227 | s/f | |
| UBQLN2 | Ubiquilin 2 | Protein degradation | 228 | s/f | May also be involved in FTD |
| VAPB | VAMP-associated protein B and C | ER-membrane protein | 229 | f | |
| VCP | Valosin containing protein | Transitional endoplasmic reticulum ATPase | Ubiquitin segregase | 230 | s/f | May also be involved in FTD |
| SBMA | |||||
| AR | Androgen receptor | Steroid hormone receptor | 231 | f | CAG repeats longer than 36–37 causes SBMA |
Abbreviations: ALS, amyotrophic lateral sclerosis; DNA, deoxyribonucleic acid; FTD, frontotemporal dementia; RNA, ribonucleic acid; SBMA, spinal and bulbar muscular atrophy; UPS, ubiquitin proteasome system; VAMP, Vesicle associated membrane protein.
Recent studies showed that about 50% of fALS cases are associated to an expansion of a hexanucleotide (G4C2) repeat located in the 5’-untranslated region of the C9ORF72 (chromosome 9 open reading frame 72) gene. This expansion generates five different highly hydrophobic dipeptides (DPRs) via a novel mechanism named repeat-associated non-ATG (RAN) translation (239–241). The DPRs, like misfolded proteins, aggregate as insoluble inclusions, and perturb intracellular processes causing neurotoxicity (194, 195, 232, 239, 242–245).
Nonneural cells involved in ALS.
Although ALS is a motoneuron disease, it is now considered a complex multifactorial disease that may involve other cell types (ie, skeletal muscle cells (246–248), astrocytes (249–251), oligodendrocytes (252), and Schwann cells (253, 254)). Reactive microglia is generally present in the areas where the disease is manifest (255), underlining the engagement of neuroinflammation and oxidative stress in the pathogenesis of the disease (256).
Sex differences in ALS.
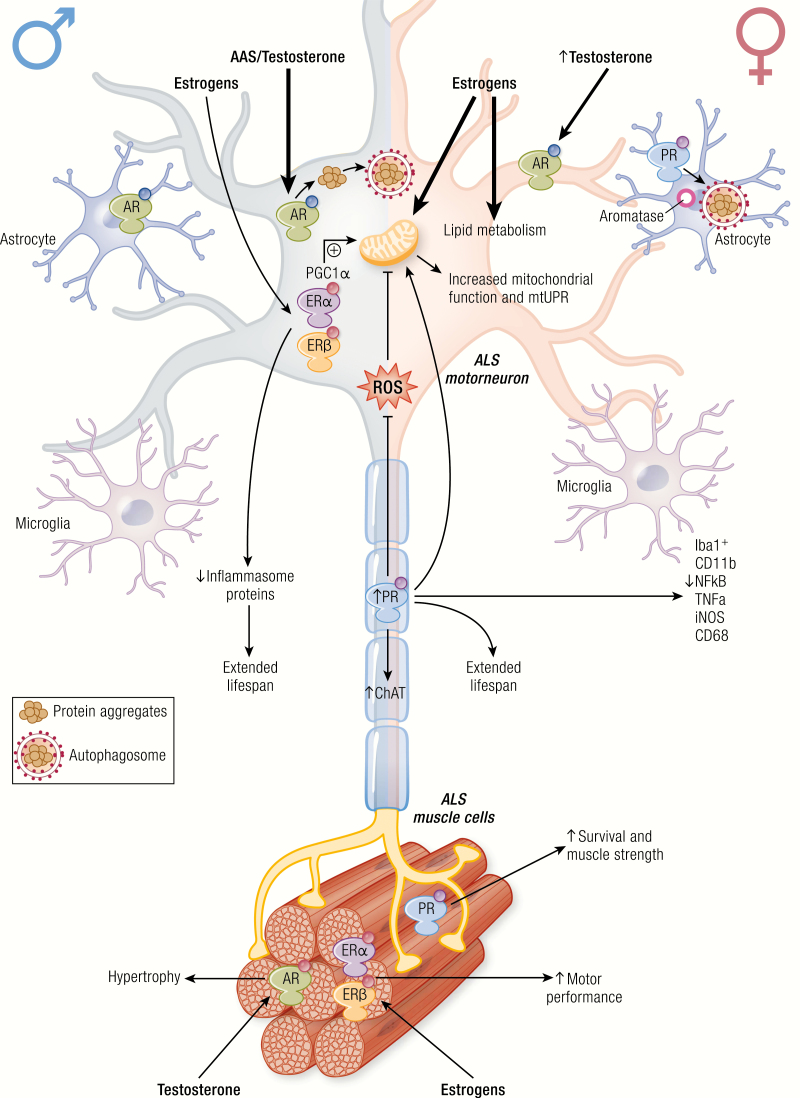
ALS is characterized by high variability in the age of onset and disease progression (Table 1). Even if the overall survival is similar in the two sexes, the disease appears earlier in males than females and with different symptomatology. In males, ALS initiates in motoneurons of the lumbar tract of the spinal cord, while in females ALS tends to begin in bulbar regions (see (38) for extensive review). The male/female ratio is between 1 and 3, depending upon the geographic area, the population considered, and the age of disease onset (254, 257, 258). A potential biological marker for the sex difference is mutant SOD1, whose concentration is dysregulated in the cerebrospinal fluid (CSF), and it is higher in male than female ALS patients (41). This difference was not found in other patients, suggesting a specific alteration of SOD1 metabolism in the two sexes (41). In addition, a retrospective study, based on executive memory and language functions in ALS patients, indicated the presence of a greater executive impairment in female than in male patients. ALS females show a 2.6-relative risk for impaired executive functions (lower scores in ALS females in phonemic fluency, trial making, and the Wisconsin card sorting test) compared with male patients, indicating an increased vulnerability in cognitive functions in female ALS patients (39). These epidemiological studies suggest that circulating estrogens may protect from some ALS alterations, while androgens might facilitate the manifestation of the pathology (259).
The molecular bases of sex-dimorphism in ALS are unknown at present. Sex steroids might directly influence specific protective or detrimental mechanisms involved in disease or determine the sex prevalence, also in relation to brain asymmetry between sexes, as further discussed. A recent study performed on a large number of ALS women with a natural menopause and well-defined oral contraceptive usage has shown a positive association between a longer reproductive condition, the susceptibility to ALS, and the survival of ALS patients, demonstrating that the longer exposure to female hormones has neuroprotective effects on motoneurons in ALS (40). Estrogens in both sexes might directly affect spinal cord motoneurons. Indeed, in the ventral horn of the lumbar spinal cord of adult mice, cytoplasmic aromatase, and nuclear estrogen receptors (ERs; ERα and ERβ) both colocalize with the motoneuron specific marker SMI-32, and with GPR30 (see following discussion) (260); thus, estradiol and phytoestrogens (which are neuroprotective in ALS mouse models (261)) may directly act as protective agents in spinal cord motoneurons.
Apart from hormonal milieu, other genetic factors may determine gender difference in ALS onset and progression. C9ORF72 or cytosine–adenine–guanine (CAG)/polyglutamine (polyQ) tract expansions (eg, in the ataxin-2 or the androgen receptor [AR]) may play a role in ALS. Of note, AR is highly expressed in spinal cord motoneurons and, when mutated with an expanded polyQ, causes an ALS-like form of motoneuron disease (see the following discussion) (193, 262). In addition, sex is a crucial factor in the C9ORF72-linked ALS, since C9ORF72 expansion negatively impacts on survival time in men but not in women: fALS male patients carrying the C9ORF72 expansion characterized by spinal onset have a reduced survival rate compared to female with the same type of onset (263). Thus, this patient cluster may be more sensitive to adverse AR action as a disease modifier in males (264).
Animal studies also support the existence of a sex-dependent susceptibility to ALS. Studies in the classical animal models of ALS, transgenic (tg) G93A-SOD1 mice or rats (265), showed that the disease is significantly more aggressive in males than in females (32, 37). In fact, male ALS mice lose weight and show motor symptoms earlier with faster symptom progression than females (32, 33). Surprisingly, sexual differences are exacerbated by the strain utilized (266) and allelic variants of chromogranin B (CHGB) gene might act as ALS disease modifiers in a sex-dependent manner (34, 267). Chromogranin is an important component of the secretory vesicles and binds to mutant SOD1 proteins, acting as a chaperone and promoting their secretions from neurons. Ohta and colleagues showed that the co-expression of CHGBL413 allelic variant in SOD1G37R mice results in pathological changes and earlier disease onset specifically in female mice (34). These differences may be due to an SRY silencer element of the CHGB promoter, which allows higher neuronal expression of CHGB in females compared to males. In patients, the sex-related effects of CHGB variants on ALS onset is still controversial: while CHGB variants are linked to an earlier disease onset in females of cohorts of Japanese and French-Canadian origins, no effect is observed in French, Swedish (34), or Italian ALS cohorts (268).
The neuroinflammatory response in ALS-affected regions and its possible correlation with the gender differences.
Neuroinflammation, together with oxidative stress, are among the main pathogenetic mechanisms for ALS (256). Studies in autoptic tissues from ALS patients or in spinal cord of tg ALS mice (255, 269–271) showed local activation of microglia (positive for the markers CD11b, Iba1, and CD68), astrocytes (glial fibrillary acidic protein [GFAP] and ALDH1L1 positive cells), and lymphocytes. In addition, spinal cord astrocytosis spread from the ventral horn (the site primarily affected) to the dorsal horn and to the sites in which the corticospinal tract fibers enter the gray matter (272), while microgliosis was present in the corticospinal tract, and in the spinal cord ventral horn where microglia interacts with T-cell infiltrates (270). In the brain, astrocytosis was detectable in the motor cortex and in other brain regions in the cortical gray matter (273) and subcortical white matter (274), while microgliosis was present in the motor cortex and in the motor nuclei of the brainstem (see (255) for extensive review). ALS mouse models analyzed pre or postsymptomatically showed that the presence of activated microglial cells anticipated the disease clinical manifestation and motoneuron loss (271, 275), while astrocytic activation paralleled motoneuronal death (276). During disease progression, microglia and astrocytes activation increased (275, 277) in parallel with an upregulation of the expression of cell-surface markers chemokines (such as CCL2) or the colony-stimulating factor 1 (255), that further contributed to microglia proliferation and activation. The current opinion is that microglia play a dual role in NDs, exerting both neuroprotective and neurotoxic functions. In ALS, microgliosis is accompanied by the activation of myeloid cells outside of the CNS (sciatic nerve) (278); T-cells (both CD4+ and CD8+) increase, and microglial activation occurs approximately at the same time in the areas involved in ALS (279, 280). So far, no studies have addressed microglia and neuroinflammation as a potential component of the sex dimorphic risk of developing ALS.
The peculiar case of spinal and bulbar muscular atrophy
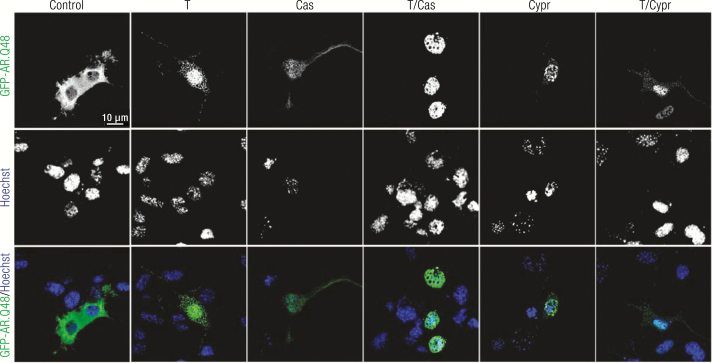
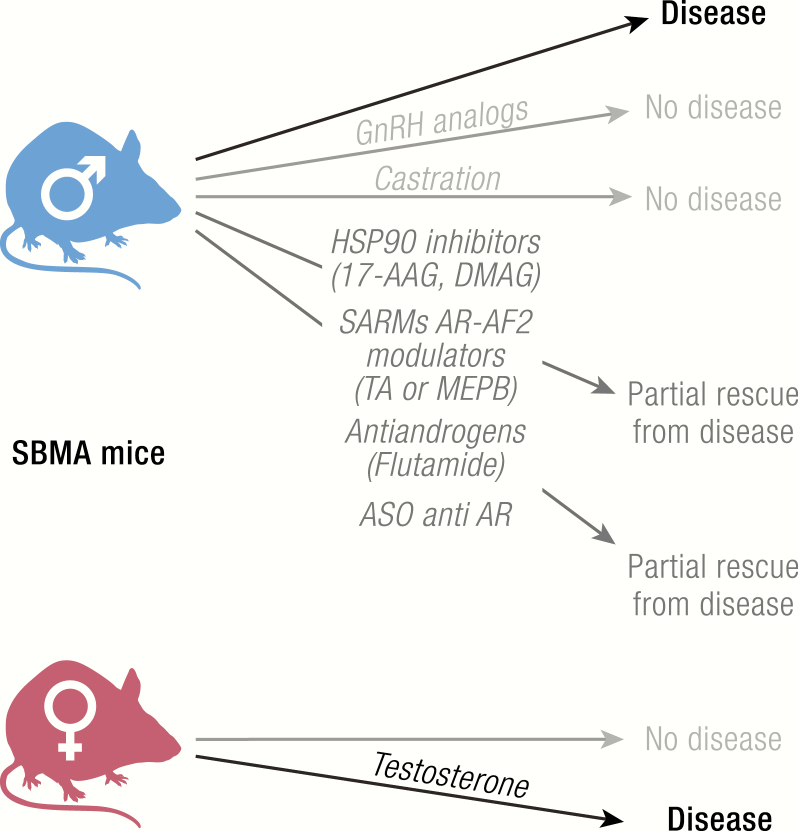
Spinal and bulbar muscular atrophy is an inherited X-linked motoneuron disease characterized by early onset (30–50 years) and a very slow progression (20–40 years). It occurs only in males (44), is caused by mutations in the AR gene, and strictly depends upon the presence of testosterone (Table 1). In fact, in all animal models of SBMA developed so far, physical or chemical castration counteracts disease onset and progression. SBMA is characterized by the loss of motoneurons in the anterior horn of the spinal cord and in the brain stem (motoneurons of the lower cranial nerves) (231, 281–284) and of dorsal root ganglia (DRG) sensory neurons (285). SBMA symptomatology includes muscle weakness and atrophy, fasciculations, dysphagia and dysarthria with atrophy of the bulbar, facial and limb muscles, and alterations in sensory function of distal extremities (283, 286). The skeletal muscle cells can also be directly affected in SBMA (285, 287–290). Endocrine dysfunctions, such as mild androgen insensitivity and alterations in the gonadal-hypothalamic axis, are part of the clinical manifestation of SBMA (291–293).
A mutation of AR, the molecular basis of the disease.
SBMA is due to an expanded CAG triplet repeat sequence in the AR gene (231). The CAG sequence is translated into elongated polyQ tract in the AR N-terminus (ARpolyQ). In the healthy population, the AR CAG repeat is highly polymorphic (15–35 repetitions) (294), with variations within human races (295); in SBMA patients, the AR CAG repeat becomes expanded from 37 to a maximum of 72 repetitions (282, 294, 296). An inverse correlation exists between polyQ size and SBMA age of onset, progression rate, and disease severity (231, 297), although exceptions to this rule (evidenced in siblings) suggest that some factors may act as disease modifiers (298). The common etiopathological denominator that associates the 9 CAG/polyQ diseases is the presence of neurotoxic intracellular aggregates of the mutant proteins (299). SBMA is not an exception to this rule, since ARpolyQ aggregates are present in the nucleus of anterior horn spinal cord motoneurons and in the cytoplasm of DRG sensory neurons of SBMA patients (300, 301).
Nonneuronal cells in SBMA.
Different from ALS, no microglia activation has been reported in SBMA. This led to the hypothesis that the very slow progression of SBMA compared to ALS is due to a lack of the inflammatory process. Notably, SBMA is now classified not only as motoneuron disease but also as neuromuscular disease. In fact, muscle biopsies from animal models and patients reveal that myopathic symptoms anticipate motoneurons degeneration and SBMA patients present myopathic symptoms not related to motoneuron degeneration (302). In a knock-in mouse model, muscle degeneration precedes the loss of motoneurons and the selective overexpression of wt AR in skeletal muscle recapitulates the disease (303). Moreover, the suppression of ARpolyQ expression exclusively in muscle ameliorates and rescues from disease (287, 288). Skeletal muscle degeneration may also be responsible for alteration of retrograde axonal transport in motoneurons (304); in addition, defects of the neuromuscular junctions are present before the motoneuron loss (305). All these observations suggest that SBMA is not a cell-autonomous disease and that both motoneuron and skeletal muscle represent primary targets of SBMA pathogenesis. This new perspective opens new potential therapeutic approaches focused not only on the CNS but also on the skeletal muscle.
Sex Steroids in the Mammalian Nervous System
Morphofunctional differences between male and female brains
Significant morphometric differences exist in the mammalian brain of the two sexes. Even after normalization for the body size, the total brain volume is significantly larger in males than females (306–308); the global structure is different, since males, compared to females, have larger hemispheres, frontal and temporal lobes, left parietal lobe, insula, cerebellum (309), amount of CSF, and volume of lateral ventricles and sulci (310). Compared to males, the female brain has a higher proportion of gray matter (310) and higher gray/white matter ratio in the frontal, temporal, parietal, occipital lobes, cingulate gyrus, and insula (reviewed in (310)). Such sexual dimorphism present at the higher organizational levels (whole brain region or selected brain regions) reflects dimorphisms at the cellular level. Indeed, neurons, neurite length, and branching of specific neuronal populations differ among sexes contributing to the dimorphism of specific brain regions. Astroglia and microglia activities differ in the two sexes impacting on neuronal response to specific stimuli or insults, including those leading to neurodegeneration. Depending of the brain region analyzed, sex-specific activities may involve dopaminergic, serotonergic, and gamma-aminobutyric acid (GABA)ergic neurons, explaining some of the sex-dependent responses to physiological or pharmacological stimuli (310–313). The existence of functional differences in cognitive abilities (eg, verbal skills or spatial abilities, reported to be better in females and males, respectively) are still object of debate (314). Studies in transsexual subjects (see (315)) showed that several sexually dimorphic brain processes change in relation to the new sexual identity. Elements of feminization can be observed in male transsexuals or masculinization in female transsexuals thus demonstrating the validity of previous observations and suggesting that specific hormonal treatments may be able to revert some of these parameters toward the characteristics of the desired sex.
The essential role of sex steroids in brain sexual differentiation
During embryogenesis and early postnatal life, brain differentiation undergoes a sexually dimorphic “organization” of the brain regions controlling gender identity (316, 317), sexual behavior, and endocrine functions (318, 319). At this developmental stage, testosterone synthesis by male gonads and its estrogenic conversion by brain aromatase are responsible for brain masculinization in males that “organizes” neural circuitries and neurons persisting for the entire life to react to circulating or locally produced steroids in a male-specific manner. This process is associated to a sexually specific expression of sex hormone receptors in numerous brain areas (3, 320). The sexual differentiation involves all neural cells: in adult brains, astrocytes show a clear sexual dimorphism (321–323) in their morphology (primary process length and number distribution) (324–326), differentiation, and function (327–330). Major microglia sex differences occur at the neonatal stage and in the adult brain in terms of abundance, distribution within CNS regions, response to exercise or stress, and expression of specific proteins (331, 332). Male microglia has a higher density and phagocytic capacity, while female microglia is more supportive of neuronal functionality. Using transcriptomics and engrafting experiments to compare the phenotype of microglia isolated from adult male and female mice, a strict association of male microglia with inflammatory processes was observed, while female microglia was associated with inhibition of inflammatory response and promotion of repair mechanisms (333).
Brain expression of steroid receptors as mediators of sex hormone activities
Sex hormone receptors.
Estrogen receptors, progesterone receptors (PRs), and ARs belong to the superfamily of hormone modulated-transcription factors. These receptors share structural homology in specific domains (eg, the central DNA- or ligand-binding domain), while the N-terminal domain and the very C-terminal tail greatly differ among nuclear receptors. After their synthesis, steroid receptors are bound to heat shock proteins (HSPs; eg, Hsp90, Hsp70, etc.) that maintain the C-terminus folded in a way to expose the ligand-binding domain. HSPs prevent activation and nuclear translocation of AR and PR by masking the nuclear localization signal and the DNA-binding domain (334, 335). Ligand-binding induces the HSPs release and receptor conformational changes, which enables posttranslational modifications (336, 337), dimerization, nuclear translocation, and binding to the specific hormone response element located in the promoter region of their target genes. This allows the recruitments of co-regulators and transcription factors to control gene transcription.
Sex hormone receptors distribution in the brain.
Brain distribution of sex steroid receptors is highly variable in function of the animal species, their developmental stage, sex, and hormonal milieu. Moreover, data have been collected both with in situ hybridization and immunocytochemistry and several differences have been found between messenger ribonucleic acid (mRNA) expression and protein production. However, it must be noted that mRNA levels do not always mirror protein levels and/or activity of any specific receptors because, in addition to transcriptional control, a complex regulation influences translation and posttranslational modifications. A brief overview of the major findings of the function of steroid receptor in the animal and human brain will be provided next.
The brain estrogen receptors.
Estrogens modulate brain functions by binding the 2 intracellular steroid receptor subtypes, ERα and ERβ (338), and their several alternative splicings identified in the human brain (339). Membrane receptors, like the G-protein-coupled receptor (GPR30), which bind 17β-estradiol with very high affinity, inducing a fast Ca++ mobilization, are also present in the brain (340, 341). Estrogens modulate several neural functions, like mood, anxiety, fear, and higher order cognitive functions, by enhancing learning and memory (342). In mammalian brain, ER distribution is generally similar throughout species (343). In humans, ERs are mainly expressed in limbic-related areas, but the 2 isoforms localization may differ. Indeed, ERα mRNA is highly expressed in the hypothalamus and amygdala, while the ERβ mRNA is highly expressed in the hippocampal formation, entorhinal cortex, and thalamus (344). In mice and rats, the ERα isoform is predominant in the preoptic area, in most of the hypothalamic nuclei, and in the hippocampus, while ERβ is predominant in the olfactory bulb, cerebral cortex, septum and preoptic area, bed nucleus of the stria terminalis (BST), amygdala, paraventricular hypothalamic nucleus, thalamus, ventral tegmental area, SN, raphe, locus coeruleus, and cerebellum. Both ERs are expressed by all neural cells (345), including microglia (346). ERs are differentially expressed in neural cells of male and female rodents: ERα is higher in females than males in the hypothalamic ventromedial nucleus, the periventricular and medial preoptic areas (347, 348), the periaqueductal gray neurons (349), and the BST (350). Both isoforms are higher in the hippocampus of females rats compared to male rats (351).
In humans, ERα is higher in women in the diagonal band of Broca and in the medial mammillary nucleus, the suprachiasmatic nucleus, and the ventromedial nucleus. Conversely, ERα levels are higher in men in the sexually dimorphic nucleus of the medial preoptic area, paraventricular nucleus, and lateral hypothalamic area. ERα is present in neurons, astrocytes, plexus choroideus, and other nonneuronal cells with some areas characterized by dimorphic distribution (348).
In the spinal cord, ERα and ERβ are present mainly in neurons in dorsal horn and in the area X and at lower levels in lumbar and sacral spinal cord (see (260)). In mice, ERα and ERβ found in SMI-32–positive anterior horn spinal cord motoneurons colocalize with aromatase and the GPR30 (260). This localization correlates with the estrogenic-induced improvement of locomotor function after spinal cord injury or in ALS (260). Estrogens neuroprotection is also recapitulated in motoneuron cell lines and in cultured facial or spinal cord motoneurons (260).
By the availability of a transgenic model designed to induce the expression of the reporter gene luciferase under the control of specific ER elements inserted into a basal promoter (the ER elements–luciferase reporter mice) (352), it was possible to quantify in which brain region of living animals estrogens transcriptionally activated ERs. This system enabled to demonstrate the presence of the sexually dimorphic ER activity in adult brains. In females, ER transcriptional activity is particularly elevated in the arcuate, hypothalamus septum, and amygdala; very little activity is observed in the motor areas (eg, striatum and SN). When female at proestrus were compared to males, significant differences in ER activity were found. In females, ER activity was significantly higher than in males in the arcuate, hypothalamus, thalamus, and septum. Interestingly the brain area with the relatively highest ER activity was the amygdala (353).. Notably, brain ER activation is associated with both circulating estrogens and, particularly in males, locally produced estrogens from circulating androgens via aromatase and 5α-reductases (5α-R)/3β-hydroxysteroid dehydrogenase (HSD)-mediated conversion, which produces 17β-estradiol (E2) and 3β-diol, a selective ERβ ligand (354) (see the following discussion for details).
The ERs differential localization and activity explain several sex-dimorphic brain functions including hypothalamic gonadotropin-releasing hormone [GnRH] release (355), hippocampal synaptic plasticity (356), neuroprotection against neurotoxic insults (357), pain modulation (358), energy metabolism regulation, the sensitivity to oxidative stress (27), the neuroinflammatory response (3), and the neuroprotection exerted in several NDs and in brain and spinal cord injuries (260).
With regards to GPR30, in rat brain in situ hybridization showed its presence in the cortex (endopiriform nucleus, motor, and somatosensory), hippocampus (CA1–CA3, dentate, and subiculum), habenula, hypothalamus (arcuate, paraventricular, suprachiasmatic, ventromedial, central, dorsomedial, and ventromedial hypothalamic nuclei) and in the SNpc (359). In these regions, GPR30 is expressed in neurons (360, 361) and in astrocytes (362), while in mouse spinal cord, GPR30 is found mainly in anterior horn motoneurons (260).
The brain progesterone receptors .
Two forms of PR, a full-length (PR-B, 110 kDa) and a N-terminally truncated form (PR-A, 86 kDa), are derived by alternative transcription of the same gene (363, 364); splice PR variants have also been described (365, 366). PR-A/PR-B expression ratio varies in the different CNS regions (367), and it is influenced by hormonal variations, age, and estrous cycle after sexual maturity (364, 368). In rodent brain, no PRs sex-dimorphism is observed. The PR is highly expressed in the BST, the centromedial amygdala, the preoptic area, the ventromedial and dorsomedial nucleus of the hypothalamus, and the arcuate nucleus of female rats (369), as well as in the norepinephrine neurons of the nucleus tractus solitarius of the brainstem (369). In ovariectomized (OVX) female mice, 17β-estradiol increases the expression of PR-B in the preoptic area, of PR-A in hippocampus and olfactory bulb, and of both PRs in the hypothalamus; no changes are present in the cortex and cerebellum (369, 370). In male rats after gonadectomy PR-A mRNA highly accumulates in the cerebellum only (369, 370). The lack of estrogen regulation in male rats is unexplained. However, since PR expression is regulated by estrogens, the brain area–selective regulation of PR expression could be due to different ERs and co-regulators expression in the various brain nuclei. The sexually dimorphic regulation of PR isoforms in the hypothalamus and preoptic area is functionally relevant for the control sexual behavior (371, 372) and anxiety (373), as well as for the production of somatostatin (374) and oxytocin receptors (375). At cellular levels, PR mRNA is present in neurons (376), in newborn rat primary cultures of CNS-derived oligodendrocytes and astrocytes (377, 378), and in peripheral nervous system (PNS)-derived Schwann cells. No PRs expression has been found in microglia (378, 379). Notably, progesterone-binding activities as also been associated with the cell membrane (380–383). In female brain, PRs mainly control reproductive behavior (384–386) and are involved in the regulation of myelination and its repair after traumatic injury, neurogenesis and regeneration, inflammation, cognitive functions, and mood (387–389).
The brain androgen receptor .
The AR gene is located on the X chromosome (390). Thus, a single AR allele exists in males, and females also utilize only one AR allele because of X chromosome inactivation occurring randomly in each cell. The AR N-terminal region is encoded by exon 1 and contains the polyQ and proline or glycine stretches (231); the DNA-binding domain and the C-terminal domain are homologous to those of ERs and PRs (391). The polyQ length is variable among individuals also in relation to ethnic backgrounds (281), and if it becomes longer than 37Q, it causes SBMA. AR is activated by testosterone and its more potent derivative dihydrotestosterone (DHT) (392–396); both steroids positively regulate AR expression (397).
Androgen receptors are expressed both in the CNS and PNS. In humans, AR is concentrated in specific hypothalamic nuclei, in the horizontal diagonal band of Broca, and in neurons of the lateromammillary nucleus, the medial mammillary nucleus, the sexually dimorphic nucleus of the preoptic area, and the infundibular nucleus. AR content is relatively lower in the BST, medial preoptic area, dorsal and ventral zones of the periventricular nucleus, supraoptic nucleus, and nucleus basalis of Meynert. AR is generally expressed at higher levels in males than in females, particularly in several hypothalamic regions, where androgens organize the male hypothalamic–pituitary–gonadal axis (see (398) for details) (399) to regulate sexual dimorphic functions (400) and might be responsible for the control of sex-dependent behaviors or for the appearance of selected psychiatric and neurological diseases, whose prevalence is sex-related (398). No major sex differences in AR expression are present in the hippocampus and in the temporal cortex (401), which are rich in AR in both sexes. In the spinal cord, AR localizes in somatic motoneurons of the anterior horn and the bulbar regions, which directly connect to striatal skeletal muscle (284). AR is also present in the DRG sensory neurons, which connect peripheral sensitive regions to posterior areas of spinal cord. Upper motoneurons in the brain motor cortex do not express AR (334). In rodents, AR localizes in somatic motoneurons of the trigeminal, facial, ambiguous, and hypoglossal nuclei (391, 402, 403), which are androgen-target cells (10, 283, 284, 301, 334, 404–407). Here, AR regulates the maturation of male motor functions, inducing neuromuscular junctions, regeneration after resection, adult dendrite, and axon growth and plasticity (334, 391, 403, 408, 409).
In conclusion, the dimorphic expression of brain sex steroid receptors may explain several sex-specific behaviors. At present time, we are unable to discriminate the extent to which gonadal and brain-derived steroids play a role in the sex specific activities controlled by the brain.
Neurosteroids and neuroactive steroids
In the brain, sex steroid receptors are mainly activated by the circulating sex steroids produced by the gonads and, to a less extent, by the adrenal gland, which freely enter the blood–brain barrier. In adult males, the circulating androgen levels are relatively constant with limited circadian, seasonal, and annual fluctuations. In humans, an androgenic peak occurs around birth and lasts for the first month of life, and then androgen levels become very low until puberty when they rise to high levels, which gradually decline with age. However, in aged males, androgen levels are significantly higher than in aged females. In males, circulating estrogens and progestins are low; however, both steroids could be synthetized in cells expressing aromatase and even secreted into the blood in endocrine dysfunctions (eg, estrogens in some feminized individuals). In females, circulating levels of estrogens and progestins are very low prior to puberty. After puberty, estrogens and progesterone synthesis is strictly controlled during the menstrual cycle with a great increase in case of pregnancy and lactation. Postmenopause, female sex steroid levels are very low. Androgen levels are generally very low, except for specific pathological conditions (polycystic ovary, hirsutism, etc.). In both sexes, all circulating sex steroids may reach the brain to participate in the regulation of their cognate receptors. Alternatively, sex steroids can be locally converted to more active metabolites as well as to compounds showing different biological activities. In addition, both CNS and PNS de novo synthetize steroid hormones locally from cholesterol (410); these steroids are indistinguishable from circulating steroids (Fig. 1). CNS/PNS synthesized steroids are named “neurosteroids” to be distinguished from the gonadal sex steroids that enter the brain via the blood stream and called “neuroactive steroids.” Several steroidogenic enzymes are present in all cell types of the CNS/PNS, and neurosteroid synthesis is the result of a coordinated interaction between neurons, macroglia, and microglia (see Fig. 1 for a detailed view of the different processes). CNS/PNS steroid synthesis might be an adaptive mechanism following brain damage (411) and neurodegenerative conditions, which usually inversely correlate with CNS/PNS steroid levels (412). The synthesis and regulation of neurosteroids and neuroactive steroids is summarized in Fig. 1. An overview of the complex distribution and activity of the various enzymes involved in sex steroids synthesis and metabolism in the brain is reported in the following disusssion.
Local activation and de novo synthesis of steroids in the brain.
Cholesterol cannot cross the blood–brain barrier, thus, it has to be locally produced (413). The enzyme essential for cholesterol synthesis, 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA red), which converts HMG-CoA into mevalonate, is expressed by all neural cells, with highest levels in astrocytes (Fig. 1). Neurosteroids biosynthesis starts with the internalization of cholesterol into mitochondria mediated by the transduceosome (414), a protein complex composed of the translocase translocator protein (TSPO; or peripheral benzodiazepine receptor) and the steroid acute regulatory protein. TSPO is present both in neurons and activated glial cells (415, 416) and microglia, which is activated by TSPO ligands (417–419). TSPO upregulation in glia is a major hallmark of brain injury, inflammation, and neurodegeneration (420). Because of this, TSPO is a marker widely exploited by positron emission tomography (PET) imaging to investigate the dynamics of microglia activation in NDs (421).
Cholesterol conversion to pregnenolone is a rate-limiting step. Pregnenolone is substrate either of the mitochondrial enzyme P450scc (and its human counterpart Cyp11a1), that generates progesterone or Cyp17, which produces dehydroepiandrosterone (Fig. 1). In rodents P450scc localizes mainly in the white matter and is more expressed in females than in males (422–425). P450scc is also elevated in the cerebral cortex, hippocampus, midbrain, and amygdala (426–428). The hortologue, CYP11A1, appears to have a similar distribution in the human brain (422, 429).
3β-hydroxysteroid dehydrogenase (3β-HSD) is responsible for progesterone synthesis. Several 3β-HSD isoforms were identified in rodents (4 in rats and 6 in mice) (430), while in humans only 2 are present: type I (mainly expressed in the placenta) (431) and type II (expressed in the gonads, adrenal gland, and brain) (432, 433). In humans 3β-HSD mRNA is detectable in the striatum, cortex, amygdala, midbrain, thalamus, hypothalamus, and cerebellum (429). Astrocytes mainly produce the 3β-HSD derivatives. In the rodent brain, the isoform 3β-HSD-1 is the mostly expressed in high concentrations in olfactory bulb, striatum, cortex, thalamus, hypothalamus, habenula, septum, hippocampus, and cerebellum (434, 435). 3β-HSD mRNA is present in neurons, astrocytes, and oligodendrocytes in primary culture (436, 437) and in Schwann cells (438).
Androgen and estrogen synthesis requires P450c17 (CYP17) (439–441), generally via dehydroepiandrosterone (442). P450c17 is highly expressed during brain development (426, 443); in adults, P450c17 is present in hippocampus astrocytes and neurons (437, 444) but not in microglia (418).
The 17β-hydroxysteroid dehydrogenase (17β-HSD) converts androstenedione and estrone to testosterone and 17β-estradiol, respectively (Fig. 1). Different 17β-HSD isoforms exist (445) that differ in tissue and subcellular localization (446–448). Type I 17β-HSD, which mainly catalyzes the activation of estrone to estradiol (439), is present in glia of mammals and amphibians (448–450); its expression is induced by inflammatory stimuli (such as LPS) in primary murine microglia cultures (418). In rats, type I 17β-HSD is widely distributed in the CNS, being localized particularly in the hippocampus, cerebral cortex, thalamus, and hypothalamus. In the humans, type I 17β-HSD is detectable in the hippocampus (451) and temporal lobe (452). Type III and Type V are the androgenic forms of 17β-HSD (439), and their expression have been detected in the hippocampus and cerebellum of human brains. Interestingly, a type X 17β-HSD is present in human cerebral cortex (453) and is potentially involved in AD pathogenesis as it binds to Aβ (454) and is upregulated in AD (455).
The previously discussed enzymes are mainly responsible for the local neurosteroids production. A second group of enzymes has a catabolic role and is capable of converting circulating sex steroids into products with higher (activatory enzymes) or different biological activities. An example is the enzyme 5α-R (Fig. 1), which converts testosterone to its more potent derivative 5α-DHT and progesterone to 5α-dihydroprogesterone (5α-DHP). DHT is the precursor of the 3β-diol, which has no androgenic activity but acquires a potent estrogenic activity (mainly mediated by ERβ binding). Instead, the 5α-DHP serves to produce allopregnenolone or 3α-hydroxy-5α-pregnan-20-one or 3α,5α-tetrahydroprogesterone. 5α-DHP, but not allopregnenolone, binds PR; both 5α-DHP and allopregnanolone bind the GABAa receptor (456). Two isoforms of 5α-R (named type 1 and type 2) have been identified (see (457–459)) with different biochemical properties but superimposable activity. 5α-Rs reduce several androgens, progestogens, and corticosteroids 3-keto-Δ4-steroids. 5α-R type 1 is expressed both in neuronal and glial cells (459), while 5α-R type 2 is confined in neuronal cells (459, 460) in specific brain regions, mainly in the neuroendocrine structures (461), including in GnRH-secreting neurons (293) and, at lower levels, in the hippocampus. 5α-R type 2 mRNA is present in anterior horn spinal cord motoneurons (407), which also express considerably levels of AR. Instead, very low levels of type 2 mRNA are present in the amygdala, olfactory bulb, and in the cerebral cortex (461). The temporal expression of the two 5α-Rs isoforms considerably differs indicating changes of their functional role from ontogeny to adulthood. 5α-R type 2 mRNA is undetectable during rat embryonic brain development at day 14; it appears at day 18 and increases to reach a maximal level at postnatal day 2 (459). This expression pattern parallels the rate of testosterone production from fetal and neonatal testis (461). Similar changes occur for aromatase mRNA expression but not for 5α-R type 1. Studies in cultured neurons confirmed that both 5α-R type 2 and aromatase, but not 5α-R type 1, are regulated by androgens.
Aromatase converts androgens to estrogens (Fig. 1). A single gene (CYP19) encodes aromatase under the control of multiple cell/tissue specific promoters, which codes different RNAs spliced at the first exon, or 5’-untranslated region to a common splice junction immediately upstream of the AUG translation initiation site, giving an identical aromatase protein. Thus, the promoter confers tissue-specific regulation to the aromatase gene (462), and those specific for the brain are 33 kb upstream of the common splicing junction.
In women, brain aromatase activity is modulated by circulating estrogens and fluctuates with the menstrual cycle (463). In rat male brain, testosterone upregulates aromatase expression. Recent studies by PET-based imaging in the baboon brain showed that aromatase is not evenly distributed in mammalian brain as the highest uptake of the aromatase binding [11C]vorozole in the amygdala, lower level in the preoptic area and hypothalamus, basal ganglia, and cortical areas (463). Aromatase is expressed in human cerebrovascular endothelium and has a sex dimorphic role in the protection against stroke (464, 465), since aromatase expression correlate with neuroprotection. Indeed, mild neurodegenerative stimuli induce severe neurodegeneration when the enzyme is genetically or pharmacologically inhibited (466), possibly because aromatase serves to locally produce neuroprotective estrogens (463, 466). In adult mice, aromatase has also been found in motoneurons of lumbar ventral horn of the spinal cord, and it is in association to nuclear ERα and ERβ expression and with GPR30 cytoplasmic and neurite localization (260). Since ALS is more frequent and appears earlier in men than women (see the following discussion) and gender difference exists also in tg ALS mice (9) with OVX mice showing an exacerbation while estrogen treatment delays symptoms progression in the same mice (467–469). This evidence suggests that estrogen may play a protective role in motoneuron NDs and particularly in ALS.
Sex steroid effects on neural cells
Estrogens are extremely pleiotropic in their activities. ERs are present in all neural cells and estrogens regulate several molecular pathways, some of which involved in ND. In neurons, estrogens prevent neuronal death by increasing the endogenous synthesis of antiapoptotic molecules (470) and the neuronal expression of growth factor receptors (471). Estrogens also improve bioenergetic activity of neurons, enhancing the ATP levels, the mitochondrial membrane potential, and the basal mitochondrial respiration (472); maintain the number of multisynaptic boutons (473); and enhance synapse formation (474). In astrocytes, estradiol has neurotrophic activity as facilitates the secretion of growth factors (475, 476), represses the expression of GFAP and reduces astrogliosis (476, 477), stimulates the release of the glial APOE necessary for neurite growth (478), and increases glutamate uptake (479). Estrogens act on microglia by attenuating the response to inflammatory stimuli, maintaining mitochondria viable, promoting the turnover of damaged proteins through the proteasome, and regulating microglial proliferation (346, 480–483). These effects were extensively reviewed elsewhere (3).
Predicting the activity of male sex hormones in the nervous systems
Circulating sex steroids have a major influence on brain functions, but their effects can be largely changed by selective enzymatic modification in their structure. As an example, testosterone may lead to a variety of androgen metabolites, each endowed with biological specificity (Fig. 2). Aromatase changes radically the biological properties of the substrate by converting testosterone into estradiol (484). 5α-R transforms testosterone into the more potent AR ligand, DHT (293, 407), but 3α-HSD and 3β-HSD convert DHT into metabolites (3α-diol and 3β-diol), which signal through membrane receptors (eg, the 3α-diol with GABAa) (485) or act as estrogens activating ERs (eg, 3β-diol binds ER-β specifically) (354). Therefore, the final biological function of circulating testosterone in target cells is unpredictable. This complex action becomes even more complicated when receptor dimerization is considered, since homo- and heterodimers may be formed providing further levels of diversification to testosterone actions. To exemplify, each monomer may associate with testosterone or DHT, giving rise to three molecular species: [AR:T/AR:T], [AR:DHT/AR:DHT], or [AR:T/AR:DHT]. In the case of testosterone being metabolized by CYP19 or the complex 5α-R/3β-HSDs, the active species generated may be: [ERα:E2/ERα:E2], [ERα:E2/ERβ:E2], [ERα:E2/ERβ:3β-diol], etc. Considering that each dimer might be endowed of promoter selectivity, the response to the same hormone may be significantly different in each given cell. An example of the complexity of the response to the various steroids and their locally formed metabolites is reported in Fig. 2.
The Influence of Sex Hormones in Neurodegenerative Diseases
Sex hormones and AD
The molecular bases of female prevalence of AD remain to be elucidated. The higher AD susceptibility of women may result from a combination of organizational and activational effects of sex steroids (486). In AD models, neonatal masculinization of female 3xTg-AD mice yielded to selective, region-specific decrease in Aβ accumulation, while neonatal blockage of testosterone in male 3xTg-AD mice led to an increase in Aβ (19). Thus, the brain masculinization induced perinatally by estradiol may certainly be a factor for AD predisposition. Conversely, epidemiological studies in aging women undergoing or not HRT showed that estradiol may be neuroprotective (487), particularly when abrupt hormonal decrease occurs as in postmenopause.
Estrogen and AD.
Genetic studies suggested that an impaired ER signaling may predispose to AD but, by itself, is insufficient to determine an increased risk to develop AD. Indeed, a clear association between impaired ERs signaling and neurodegenerative processes has not been found yet. Despite reports showing an association between aromatase polymorphisms and AD (488) and a significantly lower expression of ER mRNA splice variants in AD patients—particularly in women—than in healthy individuals (489, 490), human genetic studies have failed in providing evidence of a clear association between AD and ER polymorphisms (491–495). Certainly, the Apoε4 genotype, the most established genetic AD risk factor, has a stronger association in women than in men in cases of homozygosity (110, 111). This is possibly due to an interaction between the APOε4 variant and single nucleotide polymorphisms (SNP) of the ESR1 (rs9340799, rs2234693; rs2228480) and ESR2 (rs4986938) genes (496, 497). The pleiotropic effects of estrogens could also intervene at different steps of AD pathogenesis, the first of which is certainly Aβ accumulation (see Fig. 3).
Figure 3.
Main sexual differences and activity of sex steroid hormones on amyloid plaque deposition, neuroinflammation, and neuroprotection. The accumulation of aggregates and deposits of misfolded proteins, like amyloid beta peptides, represent the main disease-specific histopathological change in the brain of AD patients. Additional hallmarks include a diffused neuroinflammation during the advanced stages of AD. There is a strong association between APOε4 genotype, the most established genetic risk factor, with sporadic AD onset. In particular, woman carrying both the homo- and heterozygous Apoε4 isoform have a higher rate of amyloid plaque deposition, while Apoε4 variant in men has marginal effects in both homo- and heterozygous subjects. An interaction between the APOε4 variant and SNPs of the ESR1 (rs9340799, rs2234693, rs2228480) and ESR2 (rs4986938) genes is possibly involved. Estrogens, by affecting the secretase pathway in neurons and the lipid metabolism in the periphery, decrease the production of beta-amyloid peptides, thus reducing Aβ accumulation in AD. Moreover, estrogens exert anti-inflammatory effects on microglia, limiting the Aβ-induced production of ROS. The beneficial effects of estrogens are lost following menopause, or after OVX. Progesterone exerts beneficial effects on neurons, but it is still unclear if this is due to an anti-inflammatory action of progesterone per se or if the effect is mediated by the progesterone metabolite allopregnanolone. In males, AD is associated with a decrease in circulating testosterone, which, in turn, could dysregulate Aβ deposition and hinder the testosterone-mediated neuroprotective actions including the regulation of spine density. The beneficial effects of testosterone and DHT are lost in androgen deficiency in the aging male (ADAM), or after ORX.
Estrogen decreases Aβ accumulation.
Estrogens decrease the generation and secretion of β-amyloid peptides (498). In rodents, 17β-estradiol reduced Aβ accumulation in AD mouse models (482, 499–502), in aromatase knockout (KO) mice (502), and after OVX (503). Several mechanisms may be involved. In neurons, the estrogen–ER complex was shown (i) to inhibit the gene expression of the β-secretase1, the enzyme required for the production of the neurotoxic Aβ peptide (504, 505); (ii) to regulate APP processing on cell membranes by affecting lipid composition and the lipid rafts; (iii) by modulating the γ-secretase and Notch signaling (506), and (iv) by increasing the synthesis of seladin-1 (selective AD indicator-1 or 3-β-hydroxysteroid-Δ-24 reductase) that is involved in the generation of detergent-resistant membrane domains that influence APP cleavage by β- and γ-secretases (507, 508). Finally, estrogens were shown to reduce TAU hyperphosphorylation (509).
By acting on microglia, 17β-estradiol enhances Aβ peptide removal by modulating the phagocytic activity of these cells. The proposed mechanism includes the modulation of the complement pathway, which is part of the innate immune system and includes proteins associated with the rapid recognition and internalization of pathogens, apoptotic cells, cellular debris, and misfolded proteins (510). Activated ERs were shown to bind directly to the estrogen‐responsive sequences within the promoter of a key member of the complement pathway, the complement protein C3 (511), which promotes Aβ phagocytosis and clearance (510, 512). In addition, estrogens may facilitate the turnover of oxidized or otherwise damaged proteins through upregulation of microglia proteasome activity via p42/44 mitogen-activated protein kinase pathway (513, 514) and by preventing the induction of nitric oxide (515). It is conceivable that all these activities contribute to facilitate estrogen-dependent transit from the inflammatory to the anti-inflammatory state of microglia, perhaps contributing to a slowdown in microglia aging. Following menopause, in the presence of Aβ deposits, the unrestrained activation of microglia may accelerate the aging process, thus resulting in the inability of these cells to acquire the anti-inflammatory phenotype involved in cell repair. This impairment in microglial function may have detrimental consequences both for microglia and surrounding cells (483). In AD models such as APP23 mice, which overexpress the human amyloid precursor protein with the Swedish mutation, ovariectomy was shown to be associated to increased activation of the microglia at Aβ deposits; ovariectomy in these mice also facilitated the progression of microglia to their reactive state. Long-term 17β-estradiol treatment counteracted these effects reducing microglia reactivity to that found in control animals (482), suggesting that estrogen anti-inflammatory effects on microglia protected the brain by limiting Aβ peptide overburden. In addition, microglia isolated from adult mice have distinct identities and functions determined by sex (333). In young healthy mice, male microglia expressed more inflammatory biomarkers, while female microglia showed a more neuroprotective phenotype (333). The landscape changes with aging, as aged brain in females experience a higher level of microglial activation compared to age-matched males, with implications for AD development (516).
Estrogen effects on lipid metabolism.
Epidemiological studies pointed to an association between metabolic syndrome and AD in women, but not in men (517). The control of cholesterol and lipid metabolism is sexually dimorphic (319, 518), and the postmenopausal reduction of circulating estrogens alters liver lipid metabolism with severe systemic consequences (319, 519, 520). Indeed, excessive circulating lipids cause neuroinflammation (521, 522) and associated cognitive impairments (523, 524); thus, a protective estrogenic effect could be exerted via reduced lipid biosynthesis (519). Moreover, in the hippocampus and cortex, estrogens stimulate APOE release from astrocytes (478) and affect the expression of the low-density lipoprotein receptor-related protein, which triggers the formation of Aβ (525).
Progesterone and AD.
Although the positive actions of estrogens on neurodegeneration are well characterized, the role played by progesterone, also in combination with estrogens, has not been fully explained yet. Progesterone exerts beneficial effects on hippocampal-dependent spatial memory (526) and on neuronal protection (526–529). These observations have not been confirmed in specific models of neuronal damage, and the role of progesterone is still controversial (530–532). In general, progesterone-induced antioxidative (533) and anti-inflammatory actions (534) have been described. In mice, after acute episode of global ischemia, progesterone prevented the caudate nucleus neuronal death by reducing lipid peroxidation (535). For the specific case of AD, progesterone, alone or combined with 17β-estradiol, has positive effects in murine AD models, in particular on glutamate-induced oxidative injury (536, 537), glucose deprivation, and ferrous sulfate and Aβ toxicity in primary hippocampal cultures (538). This protection mediated by progesterone could be associated both to classical genomic mechanisms (536) and to activation of membrane receptors (539) leading to the neurotrophin expression. However, it is still unclear whether progesterone or its metabolite allopregnanolone mediate these neuroprotective actions.
Female hormone replacement therapy and AD.
In animal models of AD (3xTg-AD), estrogen beneficial effects on parameters like Aβ accumulation, tau hyperphosphorylation, activity of cholinergic neurons in the hippocampus, working memory, and visual attention were not modified by progesterone in acute treatments but were decreased if the hormone was administered continuously. Most relevant, clear beneficial effects of the HRT were observed when the hormone administration mimicked the ovarian cycle (500, 540), and this suggested a limited effectiveness in current HRT way of administration.
Indeed, despite these large number of data indicating that estrogens reduce AD risk, the studies on the effects of HRT in postmenopause did not reach conclusive results, as some HRT after menopause did not provide a clear vision of the effects of the treatment. A beneficial effect of estrogen therapy with regard to the onset and prevalence of AD was reported (541–543), but these results were not reproduced in others (544–546). Studies in humans are complicated by differences between HRTs, the incongruity in the time at which HRT was started (547) and the use of estrogens alone or in association with progesterone. All these elements may have a differential effect on the final AD outcome. In fact, it is known that HRT initiated few years after menopause fails to provide the beneficial effects, particularly in the nervous system. Moreover, continuous progesterone treatment inhibits the E2-mediated induction of neurotrophin expression and spatial memory performance (548). The inconsistency in the published results makes it impossible to produce a definitive meta-analysis able to summarize the overall effects of HRT on cognition (549). This is in contrast with preclinical data that are quite promising and suggest the need to search for novel HRTs aimed at slowing down the progression of dementia.
Role of male sex steroids in AD.
In males, circulating testosterone decreases slowly with aging (at an estimated rate of about 2% per year) (550, 551). However, with aging, the androgen deficiency in aging males (ADAM) syndrome may occur and cause dysfunctions in the androgen-responsive tissues, including brain (551). In the brain of AD male rodents (552) and in AD patients (553, 554), testosterone and DHT were reported to be lower than in control subjects matched for age, and, indeed, AD is associated with a decrease in mood and libido (550, 553, 555, 556) (see Fig. 3). In ORX 3xTg-AD mice the Aβ accumulation in CSF and plasma is faster than in intact controls (557), and this correlates with an impairment of behavioral performance, while a protective effect was observed after replacement with testosterone or DHT (509). Besides the regulation of Aβ deposition, androgens naturally exert neuroprotective actions in brain that include their support to neuronal growth (558, 559) and synaptic function (560–562), axonal regeneration (563, 564), and protection against neuronal death (565–567). In male brain, androgens are more potent than estrogens in regulating spine density in specific regions (562, 568), action that involves the AR activation (562, 569). The androgen-mediated protective effects on neurons can be exerted also through the attenuation of the damages induced by serum deprivation (565, 566), Aβ toxicity (570, 571), and oxidative stress (567), as demonstrated by in vitro experiments. While in vitro studies were partially paralleled in vivo (572), a recent systematic meta-analysis of the available randomized controlled trials did not support a beneficial effect of testosterone treatment on cognitive functioning in men (573). The inconsistency of the trials may be due to still limited knowledges of the mechanisms by which androgens regulate AD-related processes, including Aβ production, degradation, and clearance. Nonetheless, a low testosterone level is a risk factor for AD, and androgen replacement is still an attractive therapeutic strategy worthy of more extensive studies.
Sex hormones and PD
The NSDA neuronal circuit is highly responsive to sex steroid hormones. These signals induce specific, sometime opposite, responses in female and male brains, possibly because of the distinctive nature of the NSDA system and regulatory network in the 2 sexes (574) (Fig. 4).
Figure 4.
Sexual differences in the activity of sex steroid hormones on dopaminergic neurons of the NSDA system. Dopamine metabolism in the NSDA system, consisting of dopaminergic neurons that from the SN innervate the striatum (STR), triggers oxidative stress that may lead to dysfunctions in protein folding, mitochondrial activity, and quality control systems, which are pathogenic mechanisms of Parkinson’s disease. Astrocytes and microglia residing in the microenvironment of the SN and STR sustain DA neurotransmission, thus increasing oxidative reactions. Among sex steroid hormone receptors, only AR is expressed by DA neurons, both in males and females. In males (left side), SRY expression has been involved in DA metabolism, while high levels of testosterone sustain DA neurotransmission by targeting DA neurons and astrocytes, while only its conversion to estrogens activates microglia. Thus, male sex and hormones correlate with the potentiation DA metabolism. Higher levels of estrogens in females (right side) induce DA neurotransmission only through astrocytes and microglia. ERα-mediated microglia responses to estrogens provide anti-inflammatory and antioxidant effects.
Estrogens, NSDA, and PD.
Under physiological conditions, the NSDA system is widely controlled by estrogens, which trigger the commitment of neuroblasts toward the dopaminergic phenotype and stimulate growth and branching of neurites, metabolism, and activity of DA through ERα-mediated mechanisms (575, 576). Although in adulthood estrogens retain the ability to potentiate DA neurotransmission and provide neurotrophic support, differentiated TH-positive neurons do not express ERs and are thus unable to directly respond to estrogens (577, 578). Yet, ovarian ablation selectively depletes DA neurons in the NSDA pathway of primates and rodents, while estradiol replacement prevents this loss when added shortly (10 days) and not long (30 days) after ovarian surgery (579, 580). Estrogens action has been reconciled with the responsiveness of the microenvironment that surrounds the NSDA tract that is a complex cellular network made of interneurons, glia, input circuitries from other brain areas, and microglia. Only ERα is expressed in the SN, while ERα, ERβ, and GPR30 are present in the striatum (574, 581–584). This immune-neural network mediates estrogens regulatory action on NSDA plasticity and, plausibly, in PD. Indeed, higher estrogens levels inversely correlate with the severity of PD symptoms in women, while women who experienced early natural or surgical menopause show a higher risk of PD (585–588). Thus, estrogens are considered as neuroprotective agents, delaying the onset and mitigating the symptoms of PD, although confounding factors such as timing, dosage, composition, and route of administration of HRT in women may limit the appreciation of hormonal effects (589, 590).
Estrogens in animal models of PD.
As expected from clinical and preclinical evidence, estrogens were shown to reduce DA depletion induced by neurotoxins in the female striatum of rats and mice (29, 30). This neurotrophic effect is achieved predominantly by ERα and should be activated before the toxic insult, suggesting that estrogen action does not rescue neurotransmission in damaged neurons (357, 591). Estrogen responsiveness of the NSDA neural and immune network has been reconciled with organizational effects of estrogens, since early life exposure to these hormones reduces their neuroprotective effects in adult female mice following 6-OHDA injection (592). Some studies consistently showed that estrogens are ineffective in preventing DA neuronal death in the SN (27, 28), although other reports recently pointed to neuroprotective effects using the 6-OHDA model of PD (593–595) and in the MPTP-induced gut neurons degeneration (596). These contrasting results could be ascribed to the specific neurotoxic mechanisms, the duration of hormone deprivation, and the timing, posology, and drug identity used in hormone replacement regimens. It is thus still difficult to discern whether estrogens prevent neurodegeneration or rather potentiate neurotransmission of resilient neurons. Future studies will help to understand the role of estrogens in the sexual dimorphism of neurodegeneration in PD and in addressing the role of pathogenic mechanisms, such as protein aggregation, neuroinflammation or extra-nigral pathology (26).
Contrary to its action in females, estrogens administration worsens striatal DA depletion in the male NSDA system, although the local production of estrogens in male brain may have protective functions (27, 30, 597). Beyond effects related with the estrogen dose, the male regulatory network that controls the NSDA system has sex-specific properties and estrogen responsiveness, as further suggested by the observation that ERα and ERβ provide opposite effects in males in response to estradiol (591, 598). Estrogen responses in males are different from those observed in females and are thus expected to provide specific effects in PD-like conditions.
Progesterone and PD.
Exiguous are the studies on progesterone and PD. There is no evidence for the presence of PR in striatum and SN (367, 369). Clinical data related with the risk of PD and progesterone levels are not available. Only few reports have analyzed the effect of progesterone using animal models of PD, showing that low doses of progesterone are able to exert neuroprotective activities in male mice treated with methamphetamine (MA) or MPTP (599, 600), while a higher dose is necessary to obtain neuroprotective effects in OVX female mice treated with MA (577). In PR-KO mice, progesterone treatment increases DA signaling in the striatum but decreases the number of TH-positive cells in the SNpc when compared to wt mice (601, 602).
Androgens, NSDA, and PD.
AR is expressed in the SN and within TH-positive neurons, making the NSDA a direct target of androgens. Castration in adult mice causes an increase in the number of TH-positive cells in the SNpc, whereas testosterone reduced this cell population (603, 604). Interestingly, an opposite effect has been reported in young mice, in which castration strongly reduces the density of TH-positive neurons in the SNpc and impairs locomotor activity, effects reverted by DHT administration (605). This suggests that organizational effects in mature adult individuals may influence PD onset and symptomatology. However, the role of androgens in the NSDA system and PD patients is to date unclear. Testosterone deficiency is associated with PD patients, and testosterone therapy may have beneficial effects on motor and nonmotor symptoms in selected PD patients (606–609). Testosterone AR activation potentiates NSDA toxicity in adult PD animals through the increase in intracellular DA and the production of oxidized forms and radical species; these effects are not mediated by estrogens, since dutasteride, a 5α-R inhibitor that allows testosterone conversion to estrogens, protects dopaminergic neurons in MPTP-lesioned male mice (610, 611).
Sex steroid hormones and pathogenic mechanisms of PD.
Estrogens increase antioxidant responses and potentiating respiratory chain activity in the female brain, by inducing the expression of mitochondrial proteins and enzymes; androgens may also play a role (182, 183, 612). Sex hormone-dependent effects on protein clearance and autophagy in the brain have been scarcely investigated. Following brain iron overload in females, estrogens were shown to reduce autophagy and severity of neuronal injury, while dopaminergic neurons from male mice are more prone to autophagy activation and more vulnerable to toxicity (182).
Although the neuroprotective effects of estrogens in brain are well established and were shown to involve inflammatory cells, little information is available on the effects of sex hormones on inflammation in PD models (483, 613). Morale and colleagues showed that the amounts of toxic nitrites produced following MPTP injury in mice are inversely proportional to circulating estrogens (614). A recent study suggests that estrogens modify the reactivity of microglia to 6-OHDA in females, inducing a protective anti-inflammatory phenotype (595). Interestingly, enteric macrophages express ERα, while enteric nerve cells express both ERα and ERβ (615) and immunomodulatory and neuroprotective effects were observed for estrogens in the myenteric plexus of MPTP-treated mice (596). Although the enteric nervous system is receiving more attention in the understanding of PD pathogenesis, the role of estrogens in this district still needs to be defined.
Sex hormones and ALS
Sex-related molecular and biochemical alterations occurring in ALS: the role of sex steroids.
To date, very few data are available on the involvement of hormonal sex steroids in ALS. However, it must be recalled that sex steroids may exert organizational effects both on neuronal networks, as previously mentioned, also on muscle cells (see Fig. 5); these effects may be responsible for gender differences. The skeletal muscle cells differentially respond not only to the anabolic activity of androgens, which control muscle development in males, but also to estrogens and progestogens (616–618). In female, not only the levels of female sex hormones change during the menstrual cycle, but also the expression of their receptors (ERs and PR) is known to fluctuate in skeletal muscles, suggesting a direct role of these steroids in muscle physiology (619, 620).
Figure 5.
Effects of sex steroid hormones on motoneurons, glial and muscle cells of ALS models. Estrogens, progestagens, and androgens through their receptors (respectively, ERα, ERβ, PR, and AR) modulate different pathways in motoneurons and neighbor cells. Estrogens and progestagens extend the life span of ALS animal models through a reduced production of inflammasome proteins, directly on motoneuron or through microglial cells. Estrogens also act on motoneuron lipid metabolism, and mitochondrial functions. Furthermore, progestagens block oxidative reactions allowing a better mitochondrial activity. Androgens and AAS may lead to dysfunctions in protein folding. Skeletal muscle cells express sex steroid receptors, but while ER and PR activation in female triggers a better motor performance, in male an excessive activation of AR leads to muscle fiber hypertrophy and diminished survival of the ALS animal model.
Male sex steroids.
An increased ratio in the risk to develop ALS is observed in correlation with age of onset. This observation apparently reinforces the hypothesis that circulating sex steroid hormones in adulthood are more relevant than their possible priming action on the brain during development. So far, the contribution of the perinatal effect of sex steroids on masculinization of the nervous system is still controversial. Notably, Vivekananda and colleagues (621) pointed out the influence of prenatal factors in ALS development with the analysis of the ratio between the length of index and ring fingers (2D:4D ratio), which is known to be modulated by high prenatal testosterone levels in both sexes. In ALS patients, irrespective of sex, the 2D:4D ratio is lower than in controls, suggesting that higher prenatal circulating levels of testosterone may be an independent risk factor for ALS (621, 622). Androgens might affect motoneuron development and axonal regeneration after injury in both sexes (334, 409), suggesting that prenatal exposure to testosterone or its derivatives may influence motoneurons organization, thus altering their vulnerability in later stages of life. Moreover, it has been shown that motoneuron development may be affected by the exposure of its progenitor cells to androgens (334, 403). Interestingly, SOD1 mutations seem to have a significant, sex-specific effect on the proliferation and differentiation rate of these cells. As an example, studies performed in rat neural progenitor cells showed that in male, but not in female rat neural progenitor cells, mutant SOD1 decreased significantly their proliferative and differentiating potential (35). This is in line with studies where the exposure to prenatal testosterone was shown to directly influence motoneuron organization (334). However, glial cells together with interneurons also play a role in the androgen priming effects on the locomotor system. Herron and colleagues (36) suggested that a specific class of synaptic input originating from a small cluster of spinal interneurons, the C-boutons, may control the altered motoneuron excitability present in ALS. The C-bouton size is increased during disease progression in tg ALS mice. Notably, C-boutons only are enlarged in male ALS mice, further suggesting a sex-specific regulation of the interplay among motoneurons and their surrounding cells (36). The role played by circulating androgens in ALS is mostly associated with the expression of AR in the motoneurons, which has an organizational role in these cells (407, 408), and it is also suggested by the fact that neurons that do not express AR (cranial nerves III, IV, and VI) are spared by ALS (623). As it will be discussed later, wt AR has the propensity to form aggregates (exacerbated by the expanded polyQ tract responsible for SBMA (624)), suggesting that wt AR might contribute to the motoneuron distress, potentiating the negative effects of other proteins capable to acquire aberrant biochemical behavior in motoneurons and involved in sALS (in their wt form) and fALS (when mutated; eg, TDP-43, SOD1, etc.). However, so far, all efforts to correlate the length of the polyQ tract in wt AR with the manifestation of ALS have failed (262, 625). More convincing evidence of the involvement of AR in ALS is provided by epidemiological studies on the incidence of the disease carried out in veterans of the Gulf War (626)—particularly in males (627)—and in soccer players (628–630). These segments of the population are thought to be inclined to the use of synthetic androgenic/anabolic sex steroids (AAS) to increase muscle strength and resistance. Synthetic selective AR modulators have been produced and proposed as AAS and able to circumscribe AR transcriptional competence to the modulation of muscle anabolic activities. The effects of these AAS differs significantly from the endogenous androgens, and considering the very high levels of AR in motoneurons and muscle, it is conceivable that these AAS may have neurotoxic effects by inducing an aberrant ligand-AR conformation and/or transcriptional activity (631, 632) (see Fig. 5).
By analyzing whether a correlation exists between serum sex steroids and respiratory parameters in sALS, an increased level of circulating testosterone was found in female ALS patients compared to control females. In addition, while in normal individuals (female controls), the circulating levels of testosterone, dehydroepiandrosterone (and its sulfate), and progesterone significantly declined with age, testosterone levels remained elevated with increasing age in ALS patients. Notably, in ALS patients who show higher testosterone levels and lower progesterone/free testosterone ratio a faster worsening of respiratory parameters was observed (633). Recent data from the tg SOD1 ALS mouse model have shown that castration in male prolonged survival and disease duration, while nandrolone decanoate (an AAS) supplementation worsened motoneuron loss and reduced survival (634–636). It is of note that castration decreased AR levels in the spinal cord and muscle; nandrolone decanoate had the opposite effect and induced the formation of AR SDS-insoluble inclusions in muscle and a more pronounced astrocytic activation, supporting the negative role of androgens in ALS pathogenesis (634–636). On the contrary, in the same animal model AR blockage, obtained with flutamide, resulted in an accelerated disease onset that may contribute to muscle atrophy (637). These studies indicate that an impaired AR signaling, both increased or diminished, is detrimental for ALS patients. Finally, gender differences are evident in the autophagic process in muscle of tg ALS mice (248, 638, 639).
Female sex steroids.
The protective effects of female sex steroids may be owed to a dual action of these hormones: on one side, they may prevent cell death by acting directly on the cognate receptors expressed by motoneuron and muscle cells; on the other side, they may quench the inflammatory component of the disease. A very recent report of the Euro-MOTOR Consortium in which over than 650 female ALS patients and 1200 controls were analyzed by correlating hormonal exposures (reproductive history, breastfeeding, contraceptive use, HRT, and gynecologic surgical history) with ALS appearance have suggested that HRT correlates with a reduced risk of ALS (263).
Neuroprotective activity of progesterone and estrogens.
The potential direct cell protection is supported by a recent study in the tg SOD1 ALS mice where progesterone slowed down the progression of the disease and extended the life span of the affected male mice, without delaying the symptom onset. IHC analysis demonstrated that progesterone reduces motoneuron death by activating the autophagy degradation of mutant SOD1, thereby decreasing the load of the toxic protein in target cells (640). The positive effects of progesterone may also be due to its action in the astrocytes that surround the diseased neurons. In fact, treatment with the autophagy inhibitor 3-methyl-adenine (MA) abolished the protective effects of progesterone in cultured astrocytes derived from tg SOD1 ALS mice (640). Other data supporting the involvement of progesterone have been obtained in the Wobbler mice. These animals display a spontaneous recessive point mutation in vesicular/vacuolar protein sorting 54 (Vps54wr) that leads to a progressive degeneration of both upper and lower motoneurons with striking similarities to ALS (641). In this model, progesterone (i) decreased oxidative stress and mitochondrial membrane disruption in motoneurons inducing the expression of BDNF; (ii) restored choline-acetyltransferase activity and immunoreactivity in the spinal cord; and (iii) prevented motoneuron mitochondria vacuolization. In the same mice, progesterone treatment increased survival and muscle strength (642–644). Of note, Wobbler mice are characterized by low levels of testosterone in the testis, brain, and spinal cord (645), but high levels of corticosterone and progesterone in several tissues including the brain and all regions (cervical, thoracic, lumbar) of the spinal cord. The reduced progesterone derivatives (5α-dihydroprogesterone, allopregnanolone, and 20α-dihydroprogesterone) are also particularly elevated in the brain and in the spinal cord. It is unknown whether these changes in steroids levels correlate with motoneuron degeneration (645). Notably, a PR agonist (Nestorone®) was shown to counteract several of the typical abnormalities detectable in Wobbler mice, including the presence of vacuolated motoneurons, the reduced levels of choline-acetyltransferase and glutamine synthase. In the spinal cord, Wobbler mice are characterized by increased GFAP levels and astrogliosis (646), increased levels of Iba1+ microgliosis, of the microglial marker CD11b mRNA and of the nuclear factor kappa B (while inhibitor kappa B alpha is reduced), TNFα and inducible nitric oxide synthase mRNAs. All these parameters are reduced by the treatment with Nestorone®, except for inhibitor kappa B alpha, which is increased by the treatment (646), supporting the notion that progestogens may have beneficial effects in motoneuron diseases. These protective effects of progestogens are evident also in other animal models of neurological disorders (647). With regards to PR expression, by analyzing the spinal cord collected post-mortem from ALS patients, it has been shown that lumbar PR-A and PR-B and cervical PR-B mRNA expression are higher in ALS than controls. Even if PR-A and PR-B mainly localized in axonal processes and vessels (particularly in nerve roots and large arteries) in ALS compared with controls, they occasionally were found in motoneurons (648).
Also, estrogens may exert a protective role in ALS. The treatment with 17β-estradiol performed on presymptomatic or symptomatic male SOD1(G93A) mice resulted in enhanced motor performance and an increased survival of lumbar spinal cord motoneurons. This correlated with a reduced expression of the inflammasome proteins NLRP3, of the levels of activated caspase 1 and of the mature IL1beta, normally increased in the tg SOD1 ALS mice (469). Indeed, estrogens may directly target neurons and exert an antiapoptotic effect (357, 470). This is also true for the motoneurons, which express the classical ERs in female, and it was demonstrated in tg ALS mice where treatments with 17β-estradiol delayed the disease progression and OVX had the opposite effects (467, 468, 649). Further studies performed in cultured motoneurons showed that 17β-estradiol via the classical ERs increased Akt phosphorylation and, in turn, the Akt antiapoptotic signaling pathway glycogen synthase kinase 3 beta and B-cell lymphoma 2 (650) effects blocked by the antiestrogen ICI 182,780. Aromatase expression was found to be altered in the spinal cords of tg SOD1 ALS mice. Prior to ALS onset, aromatase is mainly expressed in motoneurons of the anterior horn spinal cord, but when ALS is manifested, aromatase expression is mainly present in astrocytes, and the overall levels of aromatase were found to be reduced during disease progression (651).
Estrogens and neuroinflammation in ALS.
Growing evidence suggests that estrogens may counteract neuroinflammation (see previous discussion and (652)) (Fig. 5). TNFα and IFNγ were indicated as essential mediators in the neuroinflammatory processes occurring in the ALS tissues (653), and both are increased in the spinal cord of tg SOD1 ALS mouse models (654) and in the blood of ALS patients (655). It has been shown that in male tg SOD1 ALS mice the treatment with 17β-estradiol increases the survival of motoneurons, and this is correlated with the downregulation of several component of the inflammatory response (eg, NLRP3, IL1beta, etc.), which are abnormally elevated in the spinal cord of these mice (469).
In cultured motoneuronal cells derived from rat embryos explants, 17β-estradiol reduced the proinflammatory activity of TNFα and IFNγ, an action fully reverted by the ER antagonist ICI 182,780 (650). Similar effects were also described with selective ER ligands (eg, propylpyrazoletriol and diarylpropionitrile), which prevented TNFα-induced apoptosis of the cultured motoneurons (656). Notably, estrogens induced overexpression of ERα, ERβ, or both, activating a neuroprotective feedback circuit involved in the upregulation of antiapoptotic proteins (phospho-AKT, phospho-cyclic AMP response element-binding protein, B-cell lymphoma 2, and phospho-Src) (656).
Metabolic disorders and ALS.
Several recent studies underlined the major involvement of estrogens, in the regulation of energy metabolism in specific cells and in most organs relevant for the metabolic control, including the brain (319), an aspect potentially relevant also for ALS. Indeed, studies in humans and in the tg SOD1 ALS model showed a direct correlation between spinal cord and motor cortex levels of both mRNA and protein of PGC-1α (a peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1 α, the master regulator of cell metabolic demands) and reduced symptoms and increased survival (657); the opposite was true when the PGC-1α levels were low (658). Interestingly, this effect of PGC-1α was found in male, but not in female, animals (659). In line with this observation, it was shown that the antitype II diabetes drug metformin, which induces PGC-1α production (660), exerts negative effects on the onset of neurological symptoms and on disease progression in female but not in male tg SOD1 ALS mice (661). The extent to which sex steroid hormones are involved in these sex-dimorphic mechanisms is unknown. However, it was shown (662) that, at least in the cerebral vascular endothelium, OVX increased the expression of PGC-1α (663); thus, it is likely that female mice have low PGC-1α levels in these districts.
Another interesting association was found in ALS patients with regard to cholesterol and lipid metabolism: total cholesterol, low-density lipoprotein, triglycerides, protein levels, and low-density lipoprotein/high-density lipoprotein ratios were significantly higher in men with ALS than in women or in the corresponding healthy controls. No alterations were found in glucose levels (664). More interesting is a study of ALS patients taking statins, drugs widely used to lower serum cholesterol. Women with ALS taking statins had a faster functional decline when compared to untreated women and men. Thus, a reduction of cholesterol levels, mediated by statins, had negative effects on ALS progression among females, but not males (665). Still unknown are the cell populations affected by the changes in cholesterol levels. It is possible that the effect of estrogens might be at the level of glia, microglia, or motoneurons.
A recent study on PPARγ, which regulates genes encoding mitochondrial fatty acid β-oxidation (666), demonstrated that the PPARγ transcriptional activity is increased in the spinal cord of symptomatic tg SOD1 ALS mice. This activity is correlated with the enhanced expression of the PPARγ target genes (eg, lipoprotein lipase, glutathione S-transferase α-2) involved in scavenging lipid peroxidation by-products. In parallel, the concentration of PPARγ increases in the motoneuron indicating a role of the receptor located in motoneuron in neutralizing deleterious lipoperoxidation derivatives within these cells (667). A potential involvement of sex steroid hormones in PGC-1α and PPARγ activities may occur directly in the motoneuron through a crosstalk between ERα and PPARs known to take place in other model systems (668). As lipid metabolism is most relevant also for the state of activation of microglia cells, a contribution to the final effect of estrogens cannot be ruled out. Alternatively, the effects of estrogens may be more relevant at systemic level where estrogens regulate lipid metabolism and transport at hepatic level (319, 519). Thus, estrogens may regulate neural cell metabolism by affecting the nature of the transport of lipoproteins, facilitating or impairing the release of lipids to cells of the nervous system.
Lipid peroxidation has also been implicated in ALS because of the high abundance of peroxidation-prone polyunsaturated fatty acids in the CNS accompanied by low antioxidant content. Diet modification designed to modify composition in favor of unsaturated fatty acid was shown to modify progression and survival of tg SOD1 ALS mice in a gender-dependent manner, with high unsaturated diet correlating with an accelerating effect in female. This was related to increased levels of protein carbonyl and glycoxidative modifications and of cytoplasmic 8-oxo-dG, which correlates with oxidation of mitochondrial DNA, likely linked to an increased production of mitochondrial free radical species induced by the highly unsaturated diet (669). The reasons why these effects are more pronounced in females are still unknown. However, an evaluation of the oxygen consumption data (routine respiration is 41.7% higher in females than in males at end-stage of disease) in lumbar spinal cord slices of tg SOD1 ALS mice demonstrated the presence of early mitochondrial impairment in males due to complex I (but not in complexes II and III) dysfunction. Thus, mitochondrial function seems to be better preserved in female than in male tg SOD1 ALS mice, in line with clinical parameters. In these animals, also the fatty acid profile evaluated using docosahexaenoic acid was significantly increased in female compared to male mice. In cultured neuronal cells (N2A9 cells) overexpressing mutant SOD1, a reduced activity of complex I function was identified, a function restored by 17β-estradiol. Thus, estradiol may delay mitochondrial dysfunction in female mice in comparison with males (670).
It is of interest that the ERα was found to regulate some mitochondrial matrix proteases and heat shock proteins involved in the classical mitochondrial unfolded protein response (UPRmt), a process that maintains the normal mitochondrial proteostasis (671). In particular, ERα upregulates the proteasome and the OMI, a mitochondrial intermembrane space (IMS) protease (671). Importantly, sex differences are detectable in the IMS-UPRmt of the spinal cords of tg SOD1 ALS mice; in fact, OMI and proteasome activity was found increased in female tg SOD1 ALS mice, while in male these parameters remained unchanged compared to age and sex matched control mice (671). This increased IMS-UPRmt response in spinal cord was not present in tg SOD1 ALS mice crossed with the male or female ERα KO mice, suggesting that ERα is required to mediate the activation of the OMI and proteasome activity of the IMS-UPRmt of the spinal cord (671).
Sex hormones, autophagy and proteasomal regulation.
Alternative biochemical pathways sensitive to sex-related functional alterations are those involving the intracellular degradative systems. In a recent study carried out in the mutSOD1 model of ALS, it has been demonstrated that the genes encoding proteins involved in autophagic or proteasomal regulation are differentially expressed in skeletal muscle and in the spinal cord; in fact, no mutSOD1 aggregates were present in the muscle despite that the functionality of this tissue is affected by SOD1 mutation. Furthermore, autophagy or proteasomal regulatory proteins are controlled in different manners in male and female mice. Our studies showed that at the presymptomatic stages no sex-related changes of the small HSP B8 (HSPB8), BCL2-associated athanogene (BAG) 3 and BAG1 expression, were observed in spinal cord and muscle of both tg SOD1 ALS and control mice. At symptomatic stage, HSPB8 expression was robustly increased in skeletal muscle of both female and male tg SOD1 ALS mice and in spinal cord of male, but not female, tg SOD1 ALS mice (638). HSPB8 can be induced by estrogens and enhances the autophagic degradation of misfolded proteins (672–674), including those formed during prolonged physical exercise in muscle. Since estrogens may modulate autophagy (675) and HSPB8, this may help to explain the potential sex difference of cells to catabolize misfolded proteins (638, 639, 676). Indeed, HSPB8 overexpression enhances the clearance of several misfolded proteins involved in ALS (including mutant SOD1, C9ORF72 related DPRs and the disease associated TDP-43 fragments), both in flies and motoneuron cellular ALS models (673, 674, 676–678). HSPB8 acts in conjunction with BAG3, HSP70, and C-terminus interacting protein CHIP, to serve as a potent facilitator of the autophagy clearance of aggregate-prone proteins, thus protecting from their neurotoxicity associated to their reduced clearance. In addition, drugs like colchicine and doxorubicin, capable to induce the de novo transcription of HSPB8 have been found active to reduce the accumulation of the ALS related protein TDP-43 and its disease associated fragments in a HSPB8 and autophagy dependent manner (677). In conclusion, since HSPB8 expression is strongly induced by estrogens (679), estrogens in female may exert their beneficial effects also via a stimulation of the overall autophagy process.
The case of SBMA
The role of testosterone.
The peculiarity of SBMA is that only male patients are affected; even women heterozygous for the CAG expansion in the AR gene are asymptomatic (680). The hypothesis of the involvement of the random inactivation X-chromosome, which would have spared at least 50% of motoneurons from AR toxicity, initially put forward was disproven by the identification of two women homozygous for SBMA. Both women did not show clinical symptom of ND (46). In addition, female carriers of the mutant AR allele have a skewed inactivation by methylation of the wt X-chromosome, a condition that favors the expression of mutant ARpolyQ in most cells. However, no clinical symptoms or electrophysiological signs are present in carrier females (45). Thus, the male incidence of SBMA is due to circulating testosterone. In line with this, as well as tg SBMA mouse models, where the mutant AR is randomly integrated into the genome (no link with X-inactivation), only males develop motor alteration, and these findings support the involvement of testosterone in disease (42, 43). It is now clear that testosterone confers to the ARpolyQ the capability to form aggregates that, at least under some circumstances, may be related to neurotoxic events (Fig. 6) (334). Indeed, these aggregates may form initially in an attempt to protect neurons from mutant misfolded ARpolyQ that may alter cell homeostasis (including transcriptional dysregulation), but later may directly exert cytotoxicity (334). In male SBMA mice, castration prevents ARpolyQ accumulation and ameliorates the disease phenotype, while testosterone administration to female SBMA mice induces ARpolyQ accumulation and causes disease manifestation (see Fig. 7). These observations are at the basis of the current hypothesis that testosterone is the switch transforming the ARpolyQ from a “nontoxic” to “neurotoxic” protein and for the proposal of the use of endocrine therapies in SBMA. So far, two endocrine therapies have been tested in humans: leuprorelin, a drug that reduces testosterone production from testis (an agonist of the GnRH receptor that interrupts the gonadotropin pulsatile production from the pituitary) and dutasteride (681), an inhibitor of the isoenzymes 5α-R 1 and 2 (highly expressed in spinal cord motoneurons (407)), which activates testosterone to a more potent androgen, DHT. Other studies have been based on the use of selective AR modulators, regulators of the AF-2 region of AR, inhibitors of HSP90s, and synthetic antisense oligonucleotides directed against AR and have provided promising results in ameliorating the aberrant phenotype observed in male SBMA mice (see Fig. 7 for details). However, these preclinical studies were not replicated in clinical studies performed on SBMA patients (43). A possible explanation is that SBMA patients are characterized by a large variability of the symptoms and a very slow disease progression; thus, the measurement of the efficacy of these treatments is very challenging and perhaps only with very long time of treatment one will be able to provide more convincing results (10, 682, 683). Recently, a male-to female transgender woman presented the typical SBMA symptoms, although she had been treated for 15 years with the antiandrogen spironolactone to reduce the levels of testosterone. Spironolactone is a synthetic aldosterone receptor antagonist with antiandrogen properties. It is widely used as antiandrogen for feminizing hormone therapy for its ability to compete with testosterone for AR binding, and it reduces gonadal steroidogenesis and 5α-R activity. In this case, spironolactone was able to induce ARpolyQ nuclear localization and toxicity leading to SBMA pathogenesis (684). Based on our better comprehension of the mechanisms governing intracellular receptor activities, 2 main theories have been put forward to explain the mechanism of testosterone-induced toxicity of the mutated AR form: nuclear toxicity and conformational alterations affecting protein folding abilities. Nuclear toxicity was suggested by the finding that the survival of SBMA motoneurons in culture and of SBMA mouse models was increased with mutations preventing ARpolyQ nuclear translocation or inducing its cytoplasmic retention (685). The second is that a conformational rearrangement is required for AR activation, since the long polyQ could alter proper folding. AR folding requires posttranslational modifications, including phosphorylation, sumoylation, and acetylation, which are known to contribute to ligand-dependent AR proteotoxicity (see (685, 686)). Misfolding during activation results in ARpolyQ nuclear and cytoplasmic aggregation, which sequesters chaperones, ubiquitin proteasome system components, transcription factors, etc. (624). However, ARpolyQ neurotoxicity is not directly due to aggregate formation (624, 687), since an inverse correlation exists between ARpolyQ aggregation and cell viability (624). Thus, at least in the earlier stages of their formation, cytoplasmic aggregates protect from cell death and ARpolyQ aggregation could be due to the cell attempt to segregate potentially neurotoxic protein species into a physically defined intracellular compartment. At late stages, aggregates may induce cell death by generating different neuronal dysfunctions. This process occurs when the neurotoxic protein is poorly cleared from neurons by specific degradative systems (see the following discussion) (632).
Figure 6.
Effects of testosterone and anti-androgens on ARpolyQ aggregation in SBMA cell model. Confocal fluorescence microscopy analysis on immortalized motoneuronal (NSC34) cells transfected with plasmid coding for a chimera of green fluorescent protein (GFP) and the AR containing an elongated polyglutamine tract (GFP-AR.Q48). Cells have been treated with ethanol (as vehicle control), 10 nM testosterone, 100 nM Casodex (Cas), or 100 nM Cyproterone acetate (Cypr) for 48 hours. Nuclei were stained with Hoechst (blue) (63X magnification). Scale bar = 10 µm. Aggregation of GFP-AR.Q48 is induced by testosterone and cyproterone acetate, but not by Casodex. Casodex, but not cyproterone acetate reverts the testosterone induced aggregation of GFP-AR.Q48.
Figure 7.
Ligand-dependent toxicity of mutant ARpolyQ in SBMA. Neurotoxicity of the mutant ARpolyQ associated with SBMA is triggered by the endogenous AR ligand testosterone. Pharmacological treatments (GnRH analogs) or surgical castration, which abolish testosterone production from the male gonads, completely rescue from the aberrant motor behavior phenotype and extend survival of all tg SBMA mouse models tested so far. Antiandrogens (eg, flutamide), SARMs inhibiting the AF-2 of AR, inhibitors of HSP90 also ameliorate motor behavior in the same tg SBMA mice. Similar results have been obtained using a genetic approach based on the administration of antisense oligonucleotides (ASO) against the AR mRNA (particularly active in muscle tissue). Conversely, female tg SBMA mice normally do not develop SBMA; testosterone treatment in female tg SBMA mice induces similar motor alteration to those described in male tg SBMA mice.
Interestingly, the ARpolyQ misfolding and aggregation triggered by testosterone is prevented and/or counteracted by some antiandrogens: the steroidal cyproterone acetate, a compound less potent than testosterone in furthering AR nuclear translocation, induces the formation of few and irregular ARpolyQ aggregates (Fig. 6) (strongly sequestered into p62 bodies) (687, 688). The nonsteroidal bicalutamide (Casodex®) prevents ARpolyQ nuclear translocation, permitting a more efficient cytoplasmic degradation of ARpolyQ via autophagy (Fig. 6) (687). Interestingly, both cyproterone acetate and bicalutamide reduced the formation of ARpolyQ insoluble species induced by testosterone (290, 631). Flutamide, another nonsteroidal AR antagonist, did not induce aggregate formation in in vitro models and exerts protective effects in SBMA mice models (689, 690). Similar data were found using selective modulators of the activation function-2 domain of the AR, which has been proposed to be essential for disease appearance. Recently, 2 new molecules: the tolfenamic acid and the 1-[2-(4-methylphenoxy)ethyl]-2-[(2-phenoxyethyl)sulfanyl]-1H-benzimidazole rescued from the loss of body weight and the altered rotarod activity or grip strength in SBMA mice models (691). In addition, protein misfolding and their deleterious effects triggered in cells are counteracted by different HSPs (Hsp40, Hsp70, and its C-terminus interacting protein) (692). These observations may lead to the generation of novel treatments; for example, HSPs can be pharmacologically modulated with drugs that enhance ARpolyQ degradation via autophagy activation (eg, 17-AAG, trehalose, etc.) (688, 693, 694), preventing ARpolyQ-mediated toxicity in motoneurons. Together, the data collected using the SBMA as model for NDs are a promising proof of principle demonstrating that by fully understanding the molecular mechanism(s) of activation of a given neurotoxic protein, there might be a possibility for pharmacological interventions aimed to modulate misfolded protein aberrant effects on neuronal survival. This clearly implies that each single mutant protein responsible for NDs must be specifically targeted with selective drugs modulating one of its functions associated to neurotoxicity (eg, bicalutamide, leuprorelin, or dutasteride in the case of SBMA). However, more selective drugs can be combined with compounds that modulate more general pathways (eg, trehalose) controlling protective/aberrant functions in neurons (eg, autophagy stimulators, chaperones inducers, etc.), clearly suggesting that NDs might be more efficiently counteracted by an accurate formulation of drug cocktails that may greatly enhance the activity of compounds poorly active when used as single treatment (631).
Conclusions and Future Perspectives
We know since decades that the activity of sex steroid hormones in the CNS is not only restricted to the control of reproductive functions and sexual behavior, but it ranges from affective behavior to learning, cognition, and motor functions. We also learned that sex steroids have a large potential for neuroprotection being able to prevent neuronal apoptosis, increase the synaptic interactions, and reduce neuroinflammatory processes. Yet, our ability to fully appreciate the complexity of the action of sex steroids in the brain has been impaired by the multiplicity of their cellular and intracellular targets and by the combined ability of the CNS to metabolize peripheral steroids or to synthesize its own steroids locally. As a consequence, a state-of-the-art understanding of the physiological role of sex steroid hormones is still very basic, and a number of questions are waiting for an answer: (i) Which are the effects of aging and decreased gonadal function on the synthesis of sex hormones in the CNS? (ii) How is receptor distribution controlled in the CNS? (iii) Will a change in peripheral synthesis of hormones (eg, due to menopause) result in upregulation of brain sex hormone receptors? (iv) What are the consequences of aging on the central activity of sex hormones in the two sexes? (v) Which is the influence of central sex steroid hormones on peripheral functions not strictly associated with reproductive actions, such as energy control or behavior? (vi) Are these effects sexually dimorphic? (vii) How do different receptor isoforms interact with each other? and (viii) Which is the physiopathological relevance of the sexual dimorphism of microglia?
The recent and major technological progress achieved in molecular imaging, genetic analysis, and stem cell biology enabled researchers to localize and measure the specific action of each steroid hormone in neural tissues along with the progression of a given neurological disease and provided the tools that may enable us to answer to all the previously cited questions. It is our hope that this review clearly demonstrates the potential that these molecules might have in delaying and circumscribing the progression of NDs and acts as a stimulus for the progression of studies in the field. Our aim was to provide a deep perspective of our current understanding of sex steroid mode of action and by collecting current understanding on the large variety of protective functions covered by sex steroids in the brain of healthy and diseased males and females. Indeed, the identification of a correlation between estrogens deficiencies and NDs may provide a mean for the study of the efficacy of replacement therapies, particularly in women after the menopause. Future studies should also include the use of modulators of androgen functions as these male hormones appear to potentiate the progress of neurodegenerative processes through different means. Even in this case, the endocrine therapy could act as a relevant complement of therapies aimed at modifying neurodegeneration course, and investigations in ALS or SBMA models should be directed at increasing our insights on endocrine therapies that decrease the toxicity of the activated AR.
To find answers, future studies should be aimed at better defining (i) the key physio-biochemical processes by which sex steroids modulate neuroinflammatory processes and promote neuronal synaptic functions and survival; (ii) the role played by sex steroids in neural cell interactions; (iii) the role of sex-specific genetics, epigenetics, and hormones in the onset and progression of NDs; and (iv) the relevance of brain sexual differentiation in all the previous processes.
Only a better understanding of these factors will enable the design of personalized preventive strategies and therapies aimed to reduce the continuous NDs expansion in industrialized countries.
Acknowledgments
Financial Support : National Institute of Health Grant RO1AG027713; European Union’s Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 278850 (INMiND); Fondazione Cariplo, Italy (2011-0591, 2014-0686 and 2017_0747); Fondazione Telethon, Italy (n. GGP14039, GGP19218); Fondazione Italiana di Ricerca per la Sclerosi Laterale Amiotrofica (AriSLA), Italy (ALS_HSPB8, ALS_Granulopathy, MLOpathy, Target_RAN), Italian Ministry of Health (n. GR-2011-02347198), Agenzia Italiana del Farmaco (AIFA) (Co_ALS), Italian Ministry of University and Research (MIUR), PRIN—Progetti di ricerca di interesse nazionale (n. 2015LFPNMN and 2017F2A2C5), MIUR progetto di eccellenza, Fondo per il Finanziamento delle Attività Base di Ricerca (FFABR-MIUR), Fondazione Regionale per la Ricerca Biomedica (FRRB) (Regione Lombardia, TRANS_ALS, project nr. 2015-0023), Università degli Studi di Milano e piano di sviluppo UNIMI—linea B, European Molecular Biology Organization (EMBO) short term fellowship (n. 537 – 2015); EU Joint Programme—Neurodegenerative Disease Research (JPND) project European Union’s Horizon 2020 research and innovation programme under grant agreement No 643417 (Grant ID: 01ED1601A, CureALS).
Additional Information
Disclosure summary : The authors have nothing to disclose.
References
- 1. Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77(1):10–18. [DOI] [PubMed] [Google Scholar]
- 2. Bartzokis G. Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol Aging. 2011;32(8):1341–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37(4):372–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paolicelli RC, Bolasco G, Pagani F, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. [DOI] [PubMed] [Google Scholar]
- 5. Rodríguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2016;323:170–182. [DOI] [PubMed] [Google Scholar]
- 6. Heneka MT, Rodríguez JJ, Verkhratsky A. Neuroglia in neurodegeneration. Brain Res Rev. 2010;63(1-2):189–211. [DOI] [PubMed] [Google Scholar]
- 7. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lo RY, Tanner CM. Epidemiology. Handbook of Parkinson’s disease 2013:24–39. [Google Scholar]
- 9. McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend Med. 2010;7(6):557–570. [DOI] [PubMed] [Google Scholar]
- 10. Katsuno M, Banno H, Suzuki K, Adachi H, Tanaka F, Sobue G. Clinical features and molecular mechanisms of spinal and bulbar muscular atrophy (SBMA). Adv Exp Med Biol. 2010;685:64–74. [DOI] [PubMed] [Google Scholar]
- 11. Takahashi S, Matsuura M, Tanabe E, et al. Age at onset of schizophrenia: gender differences and influence of temporal socioeconomic change. Psychiatry Clin Neurosci. 2000;54(2):153–156. [DOI] [PubMed] [Google Scholar]
- 12. Nolen-Hoeksema S. Gender differences in depression. Curr Dir Psychol Sci. 2001;10(5):173–176. [Google Scholar]
- 13. Schwarz JM, Bilbo SD. Sex, glia, and development: interactions in health and disease. Horm Behav. 2012;62(3):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yue M, Hanna A, Wilson J, Roder H, Janus C. Sex difference in pathology and memory decline in rTg4510 mouse model of tauopathy. Neurobiol Aging. 2011;32(4):590–603. [DOI] [PubMed] [Google Scholar]
- 15. Lee JY, Cole TB, Palmiter RD, Suh SW, Koh JY. Contribution by synaptic zinc to the gender-disparate plaque formation in human Swedish mutant APP transgenic mice. Proc Natl Acad Sci U S A. 2002;99(11):7705–7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halford RW, Russell DW. Reduction of cholesterol synthesis in the mouse brain does not affect amyloid formation in Alzheimer’s disease, but does extend lifespan. Proc Natl Acad Sci U S A. 2009;106(9):3502–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sturchler-Pierrat C, Staufenbiel M. Pathogenic mechanisms of Alzheimer’s disease analyzed in the APP23 transgenic mouse model. Ann N Y Acad Sci. 2000;920:134–139. [DOI] [PubMed] [Google Scholar]
- 18. Anderson GL, Manson J, Wallace R, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–17. [DOI] [PubMed] [Google Scholar]
- 19. Carroll JC, Rosario ER, Kreimer S, et al. Sex differences in β-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res. 2010;1366:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685–691. [DOI] [PubMed] [Google Scholar]
- 21. Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004;1019:24–28. [DOI] [PubMed] [Google Scholar]
- 22. Proust-Lima C, Amieva H, Letenneur L, Orgogozo JM, Jacqmin-Gadda H, Dartigues JF. Gender and education impact on brain aging: a general cognitive factor approach. Psychol Aging. 2008;23(3):608–620. [DOI] [PubMed] [Google Scholar]
- 23. Henderson VW, Buckwalter JG. Cognitive deficits of men and women with Alzheimer’s disease. Neurology. 1994;44(1):90–96. [DOI] [PubMed] [Google Scholar]
- 24. Schultz C, Ghebremedhin E, Braak E, Braak H. Sex-dependent cytoskeletal changes of the human hypothalamus develop independently of Alzheimer’s disease. Exp Neurol. 1999;160(1):186–193. [DOI] [PubMed] [Google Scholar]
- 25. Schwarting RK, Huston JP. Behavioral and neurochemical dynamics of neurotoxic meso-striatal dopamine lesions. Neurotoxicology. 1997;18(3):689–708. [PubMed] [Google Scholar]
- 26. Rodríguez-Navarro JA, Solano RM, Casarejos MJ, et al. Gender differences and estrogen effects in parkin null mice. J Neurochem. 2008;106(5):2143–2157. [DOI] [PubMed] [Google Scholar]
- 27. Murray HE, Pillai AV, McArthur SR, et al. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience. 2003;116(1):213–222. [DOI] [PubMed] [Google Scholar]
- 28. Miller DB, Ali SF, O’Callaghan JP, Laws SC. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann N Y Acad Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- 29. Gillies GE, Murray HE, Dexter D, McArthur S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol Biochem Behav. 2004;78(3):513–522. [DOI] [PubMed] [Google Scholar]
- 30. McArthur S, Murray HE, Dhankot A, Dexter DT, Gillies GE. Striatal susceptibility to a dopaminergic neurotoxin is independent of sex hormone effects on cell survival and DAT expression but is exacerbated by central aromatase inhibition. J Neurochem. 2007;100(3):678–692. [DOI] [PubMed] [Google Scholar]
- 31. Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78(8):819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cervetto C, Frattaroli D, Maura G, Marcoli M. Motor neuron dysfunction in a mouse model of ALS: gender-dependent effect of P2X7 antagonism. Toxicology. 2013;311(1-2):69–77. [DOI] [PubMed] [Google Scholar]
- 33. Oliván S, Calvo AC, Rando A, Muñoz MJ, Zaragoza P, Osta R. Comparative study of behavioural tests in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Exp Anim. 2015;64(2):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohta Y, Soucy G, Phaneuf D, et al. Sex-dependent effects of chromogranin B P413L allelic variant as disease modifier in amyotrophic lateral sclerosis. Hum Mol Genet. 2016;25(21):4771–4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li R, Strykowski R, Meyer M, Mulcrone P, Krakora D, Suzuki M. Male-specific differences in proliferation, neurogenesis, and sensitivity to oxidative stress in neural progenitor cells derived from a rat model of ALS. PloS One. 2012;7(11):e48581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Herron LR, Miles GB. Gender-specific perturbations in modulatory inputs to motoneurons in a mouse model of amyotrophic lateral sclerosis. Neuroscience. 2012;226:313–323. [DOI] [PubMed] [Google Scholar]
- 37. Stam NC, Nithianantharajah J, Howard ML, Atkin JD, Cheema SS, Hannan AJ. Sex-specific behavioural effects of environmental enrichment in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2008;28(4):717–723. [DOI] [PubMed] [Google Scholar]
- 38. Blasco H, Guennoc AM, Veyrat-Durebex C, et al. Amyotrophic lateral sclerosis: a hormonal condition? Amyotroph Lateral Scler. 2012;13(6):585–588. [DOI] [PubMed] [Google Scholar]
- 39. Palmieri A, Mento G, Calvo Vet al. Female gender doubles executive dysfunction risk in ALS: a case-control study in 165 patients. J Neurol Neurosurg Psychiatry. 2015;86(5):574–579. [DOI] [PubMed] [Google Scholar]
- 40. de Jong S, Huisman M, Sutedja N, et al. Endogenous female reproductive hormones and the risk of amyotrophic lateral sclerosis. J Neurol. 2013;260(2):507–512. [DOI] [PubMed] [Google Scholar]
- 41. Frutiger K, Lukas TJ, Gorrie G, Ajroud-Driss S, Siddique T. Gender difference in levels of Cu/Zn superoxide dismutase (SOD1) in cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9(3):184–187. [DOI] [PubMed] [Google Scholar]
- 42. Katsuno M, Adachi H, Kume A, et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35(5):843–854. [DOI] [PubMed] [Google Scholar]
- 43. Katsuno M, Adachi H, Doyu M, et al. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med. 2003;9(6):768–773. [DOI] [PubMed] [Google Scholar]
- 44. Ishihara H, Kanda F, Nishio H, Sumino K, Chihara K. Clinical features and skewed X-chromosome inactivation in female carriers of X-linked recessive spinal and bulbar muscular atrophy. J Neurol. 2001;248(10):856–860. [DOI] [PubMed] [Google Scholar]
- 45. Paradas C, Solano F, Carrillo F, et al. Highly skewed inactivation of the wild-type X-chromosome in asymptomatic female carriers of spinal and bulbar muscular atrophy (Kennedy’s disease). J Neurol. 2008;255(6):853–857. [DOI] [PubMed] [Google Scholar]
- 46. Schmidt BJ, Greenberg CR, Allingham-Hawkins DJ, Spriggs EL. Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology. 2002;59(5):770–772. [DOI] [PubMed] [Google Scholar]
- 47. Qiu C, Fratiglioni L. Epidemiology of Alzheimer’s disease. In: Alzheimer’s Disease. 2nd ed. New York: Oxford University Press; 2017. doi: 10.1093/med/9780198779803.003.0003 [DOI] [Google Scholar]
- 48. Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology. 2013;80(19):1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alzheimer’s Association. 2018 Alzheimer’s disease facts and figures. Alzheimers Dement. 2018;14(3):367–429. [Google Scholar]
- 50. Villemagne VL, Burnham S, Bourgeat P, et al. ; Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group . Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. [DOI] [PubMed] [Google Scholar]
- 51. Arendt T, Bigl V, Tennstedt A, Arendt A. Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical target areas in Alzheimer’s disease. Neuroscience. 1985;14(1):1–14. [DOI] [PubMed] [Google Scholar]
- 52. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chartier-Harlin MC, Crawford F, Houlden H, et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991;353(6347):844–846. [DOI] [PubMed] [Google Scholar]
- 54. Hollingworth P, Harold D, Sims R, et al. ; Alzheimer’s Disease Neuroimaging Initiative; CHARGE consortium; EADI1 consortium . Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat Genet. 2011;43(5):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jansen IE, Savage JE, Watanabe K, et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat Genet. 2019;51(3):404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. [DOI] [PubMed] [Google Scholar]
- 57. Boada M, Antúnez C, Ramírez-Lorca R, et al. ; Alzheimer’s Disease Neuroimaging Initiative . ATP5H/KCTD2 locus is associated with Alzheimer’s disease risk. Mol Psychiatry. 2014;19(6):682–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Seshadri S, Fitzpatrick AL, Ikram MA, et al. ; CHARGE Consortium; GERAD1 Consortium; EADI1 Consortium . Genome-wide analysis of genetic loci associated with Alzheimer disease. Jama. 2010;303(18):1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lambert JC, Ibrahim-Verbaas CA, Harold D, et al. ; European Alzheimer’s Disease Initiative (EADI); Genetic and Environmental Risk in Alzheimer’s Disease; Alzheimer’s Disease Genetic Consortium; Cohorts for Heart and Aging Research in Genomic Epidemiology . Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45(12):1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Harold D, Abraham R, Hollingworth P, et al. ;. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lambert JC, Heath S, Even G, et al. ; European Alzheimer’s Disease Initiative Investigators . Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet. 2009;41(10):1094–1099. [DOI] [PubMed] [Google Scholar]
- 62. Doran M, du Plessis DG, Ghadiali EJ, Mann DM, Pickering-Brown S, Larner AJ. Familial early-onset dementia with tau intron 10 + 16 mutation with clinical features similar to those of Alzheimer disease. Arch Neurol. 2007;64(10):1535–1539. [DOI] [PubMed] [Google Scholar]
- 63. Cruchaga C, Kauwe JS, Mayo K, et al. ; Alzheimer’s Disease Neuroimaging Initiative . SNPs associated with cerebrospinal fluid phospho-tau levels influence rate of decline in Alzheimer’s disease. Plos Genet. 2010;6(9):e1001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. [DOI] [PubMed] [Google Scholar]
- 65. Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–977. [DOI] [PubMed] [Google Scholar]
- 66. Guerreiro R, Wojtas A, Bras J, et al. ; Alzheimer Genetic Analysis Group . TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368(2):117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ruiz A, Heilmann S, Becker T, et al. ; IGAP . Follow-up of loci from the international genomics of Alzheimer’s disease project identifies TRIP4 as a novel susceptibility gene. Transl Psychiatry. 2014;4:e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wetzel-Smith MK, Hunkapiller J, Bhangale TR, et al. ; Alzheimer’s Disease Genetics Consortium . A rare mutation in UNC5C predisposes to late-onset Alzheimer’s disease and increases neuronal cell death. Nat Med. 2014;20(12):1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goldman JS, Hahn SE, Catania JW, et al. ; American College of Medical Genetics and the National Society of Genetic Counselors . Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;13(6):597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lacour A, Espinosa A, Louwersheimer E, et al. Genome-wide significant risk factors for Alzheimer’s disease: role in progression to dementia due to Alzheimer’s disease among subjects with mild cognitive impairment. Mol Psychiatry. 2017;22(1):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer’s disease: a systematic review of the evidence. Neurotoxicology. 2017;61:143–187. [DOI] [PubMed] [Google Scholar]
- 72. Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rasmussen KL, Tybjærg-Hansen A, Nordestgaard BG, Frikke-Schmidt R. Plasma apolipoprotein E levels and risk of dementia: a Mendelian randomization study of 106,562 individuals. Alzheimers Dement. 2018;14(1):71–80. [DOI] [PubMed] [Google Scholar]
- 74. Gong CX, Liu F, Iqbal K. Multifactorial hypothesis and multi-targets for Alzheimer’s disease. J Alzheimers Dis. 2018;64(s1):S107–S117. [DOI] [PubMed] [Google Scholar]
- 75. Behl C. Amyloid in Alzheimer’s disease: guilty beyond reasonable doubt? Trends Pharmacol Sci. 2017;38(10):849–851. [DOI] [PubMed] [Google Scholar]
- 76. Moir RD, Lathe R, Tanzi RE. The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimers Dement. 2018;14(12):1602–1614. [DOI] [PubMed] [Google Scholar]
- 77. Morris JC, Aisen PS, Bateman RJ, et al. Developing an international network for Alzheimer research: The Dominantly Inherited Alzheimer Network. Clin Investig (Lond). 2012;2(10):975–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Foley P. Lipids in Alzheimer’s disease: a century-old story. Biochim Biophys Acta. 2010;1801(8):750–753. [DOI] [PubMed] [Google Scholar]
- 79. Chang TY, Yamauchi Y, Hasan MT, Chang C. Cellular cholesterol homeostasis and Alzheimer’s disease. J Lipid Res. 2017;58(12):2239–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12(5):284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52(1):131–145. [DOI] [PubMed] [Google Scholar]
- 82. Mishra A, Brinton RD. Inflammation: bridging age, menopause and APOEε4 genotype to Alzheimer’s disease. Front Aging Neurosci. 2018;10:312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mosher KI, Wyss-Coray T. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochem Pharmacol. 2014;88(4):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21(2):195–218. [DOI] [PubMed] [Google Scholar]
- 85. Nixon RA, Yang DS. Autophagy failure in Alzheimer’s disease–locating the primary defect. Neurobiol Dis. 2011;43(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nixon RA, Cataldo AM. Lysosomal system pathways: genes to neurodegeneration in Alzheimer’s disease. J Alzheimers Dis. 2006;9(3 Suppl):277–289. [DOI] [PubMed] [Google Scholar]
- 87. Demuro A, Parker I, Stutzmann GE. Calcium signaling and amyloid toxicity in Alzheimer disease. J Biol Chem. 2010;285(17):12463–12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47(2):122–129. [DOI] [PubMed] [Google Scholar]
- 89. Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci. 2003;23(7):2557–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kruman II, Wersto RP, Cardozo-Pelaez F, et al. Cell cycle activation linked to neuronal cell death initiated by DNA damage. Neuron. 2004;41(4):549–561. [DOI] [PubMed] [Google Scholar]
- 91. Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimers Dement. 2014;10(1 Suppl):S76–S83. [DOI] [PubMed] [Google Scholar]
- 92. Cholerton B, Baker LD, Craft S. Insulin, cognition, and dementia. Eur J Pharmacol. 2013;719(1-3):170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang R, Li JJ, Diao S, et al. Metabolic stress modulates Alzheimer’s β-secretase gene transcription via SIRT1-PPARγ-PGC-1 in neurons. Cell Metab. 2013;17(5):685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382(6593):716–719. [DOI] [PubMed] [Google Scholar]
- 95. Bolmont T, Haiss F, Eicke D, et al. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28(16):4283–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mandrekar S, Jiang Q, Lee CY, Koenigsknecht-Talboo J, Holtzman DM, Landreth GE. Microglia mediate the clearance of soluble Abeta through fluid phase macropinocytosis. J Neurosci. 2009;29(13):4252–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu Z, Condello C, Schain A, Harb R, Grutzendler J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J Neurosci. 2010;30(50):17091–17101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J Neurosci. 2008;28(33):8354–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45(2):208–212. [DOI] [PubMed] [Google Scholar]
- 100. Giunta B, Fernandez F, Nikolic WV, et al. Inflammaging as a prodrome to Alzheimer’s disease. J Neuroinflammation. 2008;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Peters R. Ageing and the brain. Postgrad Med J. 2006;82(964):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25(5):663–674. [DOI] [PubMed] [Google Scholar]
- 103. Mazure CM, Swendsen J. Sex differences in Alzheimer’s disease and other dementias. Lancet Neurol. 2016;15(5):451–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Plassman BL, Langa KM, McCammon RJ, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70(3):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- 106. Giovagnoli AR, Erbetta A, Reati F, Bugiani O. Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer’s disease in a study of diagnostic concordance. Neuropsychologia. 2008;46(5):1495–1504. [DOI] [PubMed] [Google Scholar]
- 107. Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology. 2017;89(13):1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49(6):1498–1504. [DOI] [PubMed] [Google Scholar]
- 109. Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimers Dement. 2015;11(3):310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Altmann A, Tian L, Henderson VW, Greicius MD; Alzheimer’s Disease Neuroimaging Initiative Investigators . Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ungar L, Altmann A, Greicius MD. Apolipoprotein E, gender, and Alzheimer’s disease: an overlooked, but potent and promising interaction. Brain Imaging Behav. 2014;8(2):262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Neu SC, Pa J, Kukull Wet al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74(10):1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zokaei N, Giehl K, Sillence A, et al. Sex and APOE: A memory advantage in male APOE ε4 carriers in midlife. Cortex. 2017;88:98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Zhou ZD, Lim TM. Roles of glutathione (GSH) in dopamine (DA) oxidation studied by improved tandem HPLC plus ESI-MS. Neurochem Res. 2009;34(2):316–326. [DOI] [PubMed] [Google Scholar]
- 115. Hwang O. Role of oxidative stress in Parkinson’s disease. Exp Neurobiol. 2013;22(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95(11):6469–6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24(2):197–211. [DOI] [PubMed] [Google Scholar]
- 118. Holmqvist S, Chutna O, Bousset L, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–820. [DOI] [PubMed] [Google Scholar]
- 119. Warner TT, Schapira AH. Genetic and environmental factors in the cause of Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S16–S23; discussion S23-15. [DOI] [PubMed] [Google Scholar]
- 120. Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson’s disease: a review of the evidence. Eur J Epidemiol. 2011;26 Suppl 1:S1–S58. [DOI] [PubMed] [Google Scholar]
- 121. Ramirez A, Heimbach A, Gründemann J, et al. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38(10):1184–1191. [DOI] [PubMed] [Google Scholar]
- 122. Bonifati V, Rizzu P, van Baren MJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299(5604):256–259. [DOI] [PubMed] [Google Scholar]
- 123. Sidransky E, Nalls MA, Aasly JO, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361(17):1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Neumann J, Bras J, Deas E, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132(Pt 7):1783–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F. A new locus for Parkinson’s disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol. 2002;51(3):296–301. [DOI] [PubMed] [Google Scholar]
- 126. Valente EM, Abou-Sleiman PM, Caputo V, et al. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304(5674):1158–1160. [DOI] [PubMed] [Google Scholar]
- 127. Deng H, Le WD, Hunter CB, et al. Heterogeneous phenotype in a family with compound heterozygous parkin gene mutations. Arch Neurol. 2006;63(2):273–277. [DOI] [PubMed] [Google Scholar]
- 128. Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. [DOI] [PubMed] [Google Scholar]
- 129. Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. [DOI] [PubMed] [Google Scholar]
- 130. Venda LL, Cragg SJ, Buchman VL, Wade-Martins R. α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010;33(12):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Nakaso K, Tajima N, Ito S, et al. Dopamine-mediated oxidation of methionine 127 in α-synuclein causes cytotoxicity and oligomerization of α-synuclein. PLoS One. 2013;8(2):e55068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Williams-Gray CH, Goris A, Saiki M, et al. Apolipoprotein E genotype as a risk factor for susceptibility to and dementia in Parkinson’s disease. J Neurol. 2009;256(3):493–498. [DOI] [PubMed] [Google Scholar]
- 133. Yang Q, She H, Gearing M, et al. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science. 2009;323(5910):124–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gan-Or Z, Giladi N, Rozovski U, et al. Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology. 2008;70(24):2277–2283. [DOI] [PubMed] [Google Scholar]
- 135. Winslow AR, Chen CW, Corrochano S, et al. α-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol. 2010;190(6):1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Sala G, Marinig D, Arosio A, Ferrarese C. Role of chaperone-mediated autophagy dysfunctions in the pathogenesis of Parkinson’s disease. Front Mol Neurosci. 2016;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ebrahimi-Fakhari D, Cantuti-Castelvetri I, Fan Z, et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. J Neurosci. 2011;31(41):14508–14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Martinez-Vicente M, Talloczy Z, Kaushik S, et al. Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest. 2008;118(2):777–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–1291. [DOI] [PubMed] [Google Scholar]
- 140. Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. J Neuroimmune Pharmacol. 2013;8(1):189–201. [DOI] [PubMed] [Google Scholar]
- 141. Choi JG, Kim N, Ju IG, et al. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci Rep. 2018;8(1):1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–397. [DOI] [PubMed] [Google Scholar]
- 143. Krüger R, Hardt C, Tschentscher F, et al. Genetic analysis of immunomodulating factors in sporadic Parkinson’s disease. J Neural Transm (Vienna). 2000;107(5):553–562. [DOI] [PubMed] [Google Scholar]
- 144. López González I, Garcia-Esparcia P, Llorens F, Ferrer I. Genetic and transcriptomic profiles of inflammation in neurodegenerative diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and Tauopathies. Int J Mol Sci. 2016;17(2):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Stolzenberg E, Berry D, Yang, Lee EY, et al. A role for neuronal alpha-synuclein in gastrointestinal immunity. J Innate Immun. 2017;9(5):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Jagmag SA, Tripathi N, Shukla SD, Maiti S, Khurana S. Evaluation of models of Parkinson’s disease. Front Neurosci. 2015;9:503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Bayir H, Kapralov AA, Jiang J, et al. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome C: protection against apoptosis versus delayed oxidative stress in Parkinson disease. J Biol Chem. 2009;284(23):15951–15969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Choi J, Sullards MC, Olzmann JA, et al. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281(16):10816–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Bosco DA, Fowler DM, Zhang Q, et al. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat Chem Biol. 2006;2(5):249–253. [DOI] [PubMed] [Google Scholar]
- 150. Shimura-Miura H, Hattori N, Kang D, Miyako K, Nakabeppu Y, Mizuno Y. Increased 8-oxo-dGTPase in the mitochondria of substantia nigral neurons in Parkinson’s disease. Ann Neurol. 1999;46(6):920–924. [PubMed] [Google Scholar]
- 151. Koprich JB, Reske-Nielsen C, Mithal P, Isacson O. Neuroinflammation mediated by IL-1beta increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J Neuroinflammation. 2008;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Gerhard A, Pavese N, Hotton G, et al. In vivo imaging of microglial activation with [11C]®-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis. 2006;21(2):404–412. [DOI] [PubMed] [Google Scholar]
- 153. Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81(6):1285–1297. [DOI] [PubMed] [Google Scholar]
- 154. Armentero MT, Levandis G, Nappi G, Bazzini E, Blandini F. Peripheral inflammation and neuroprotection: systemic pretreatment with complete Freund’s adjuvant reduces 6-hydroxydopamine toxicity in a rodent model of Parkinson’s disease. Neurobiol Dis. 2006;24(3):492–505. [DOI] [PubMed] [Google Scholar]
- 155. Liberatore GT, Jackson-Lewis V, Vukosavic S, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5(12):1403–1409. [DOI] [PubMed] [Google Scholar]
- 156. Main BS, Zhang M, Brody KM, et al. Type-1 interferons contribute to the neuroinflammatory response and disease progression of the MPTP mouse model of Parkinson’s disease. Glia. 2016;64(9):1590–1604. [DOI] [PubMed] [Google Scholar]
- 157. Wu XF, Block ML, Zhang W, et al. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7(5-6):654–661. [DOI] [PubMed] [Google Scholar]
- 158. Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. [DOI] [PubMed] [Google Scholar]
- 159. Wu DC, Jackson-Lewis V, Vila M, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22(5):1763–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28(30):7687–7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Béraud D, Hathaway HA, Trecki J, et al. Microglial activation and antioxidant responses induced by the Parkinson’s disease protein α-synuclein. J Neuroimmune Pharmacol. 2013;8(1):94–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Zhang W, Dallas S, Zhang D, et al. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55(11):1178–1188. [DOI] [PubMed] [Google Scholar]
- 163. Qin L, Liu Y, Hong JS, Crews FT. NADPH oxidase and aging drive microglial activation, oxidative stress, and dopaminergic neurodegeneration following systemic LPS administration. Glia. 2013;61(6):855–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Vroon A, Drukarch B, Bol JG, et al. Neuroinflammation in Parkinson’s patients and MPTP-treated mice is not restricted to the nigrostriatal system: microgliosis and differential expression of interleukin-1 receptors in the olfactory bulb. Exp Gerontol. 2007;42(8):762–771. [DOI] [PubMed] [Google Scholar]
- 165. Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Liu Y, Qin L, Wilson B, et al. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008;29(5):864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ling ZD, Chang Q, Lipton JW, Tong CW, Landers TM, Carvey PM. Combined toxicity of prenatal bacterial endotoxin exposure and postnatal 6-hydroxydopamine in the adult rat midbrain. Neuroscience. 2004;124(3):619–628. [DOI] [PubMed] [Google Scholar]
- 168. McGeer EG, McGeer PL. The role of anti-inflammatory agents in Parkinson’s disease. CNS Drugs. 2007;21(10):789–797. [DOI] [PubMed] [Google Scholar]
- 169. Qin L, Liu Y, Wang T, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279(2):1415–1421. [DOI] [PubMed] [Google Scholar]
- 170. Sundar Boyalla S, Barbara Victor M, Roemgens A, Beyer C, Arnold S. Sex- and brain region-specific role of cytochrome c oxidase in 1-methyl-4-phenylpyridinium-mediated astrocyte vulnerability. J Neurosci Res. 2011;89(12):2068–2082. [DOI] [PubMed] [Google Scholar]
- 171. Kim BW, Jeong KH, Kim JH, et al. Pathogenic upregulation of glial lipocalin-2 in the Parkinsonian dopaminergic system. J Neurosci. 2016;36(20):5608–5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Janda E, Lascala A, Carresi C, et al. Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: implications for neuroprotection. Autophagy. 2015;11(7):1063–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Gan L, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci. 2012;32(49):17775–17787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Booth HDE, Hirst WD, Wade-Martins R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017;40(6):358–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biol Sex Differ. 2011;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Acaz-Fonseca E, Duran JC, Carrero P, Garcia-Segura LM, Arevalo MA. Sex differences in glia reactivity after cortical brain injury. Glia. 2015;63(11):1966–1981. [DOI] [PubMed] [Google Scholar]
- 177. Picillo M, Nicoletti A, Fetoni V, Garavaglia B, Barone P, Pellecchia MT. The relevance of gender in Parkinson’s disease: a review. J Neurol. 2017;264(8):1583–1607. [DOI] [PubMed] [Google Scholar]
- 178. Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, et al. Effects of gender on nigral gene expression and parkinson disease. Neurobiol Dis. 2007;26(3):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Czech DP, Lee J, Sim H, Parish CL, Vilain E, Harley VR. The human testis-determining factor SRY localizes in midbrain dopamine neurons and regulates multiple components of catecholamine synthesis and metabolism. J Neurochem. 2012;122(2):260–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Munro CA, McCaul ME, Wong DF, et al. Sex differences in striatal dopamine release in healthy adults. Biol Psychiatry. 2006;59(10):966–974. [DOI] [PubMed] [Google Scholar]
- 181. Du L, Hickey RW, Bayir H, et al. Starving neurons show sex difference in autophagy. J Biol Chem. 2009;284(4):2383–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wang LF, Yokoyama KK, Chen TY, et al. Male-specific alleviation of iron-induced striatal injury by inhibition of autophagy. PloS One. 2015;10(7):e0131224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wang LF, Yokoyama KK, Lin CL, et al. Knockout of ho-1 protects the striatum from ferrous iron-induced injury in a male-specific manner in mice. Sci Rep. 2016;6:26358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Cole TB, Coburn J, Dao K, et al. Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology. 2016;374:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Demarest TG, McCarthy MM. Sex differences in mitochondrial (dys)function: Implications for neuroprotection. J Bioenerg Biomembr. 2015;47(1-2):173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Misiak M, Beyer C, Arnold S. Gender-specific role of mitochondria in the vulnerability of 6-hydroxydopamine-treated mesencephalic neurons. Biochim Biophys Acta. 2010;1797(6-7):1178–1188. [DOI] [PubMed] [Google Scholar]
- 187. Pomatto LCD, Carney C, Shen B, et al. The mitochondrial lon protease is required for age-specific and sex-specific adaptation to oxidative stress. Curr Biol. 2017;27(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Joniec I, Ciesielska A, Kurkowska-Jastrzebska I, et al. Age- and sex-differences in the nitric oxide synthase expression and dopamine concentration in the murine model of Parkinson’s disease induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 2009;1261:7–19. [DOI] [PubMed] [Google Scholar]
- 189. Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci. 2013;14(4):248–264. [DOI] [PubMed] [Google Scholar]
- 190. Yang Y, Hentati A, Deng HX, et al. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29(2):160–165. [DOI] [PubMed] [Google Scholar]
- 191. Greenway MJ, Andersen PM, Russ C, et al. ANG mutations segregate with familial and ‘sporadic’ amyotrophic lateral sclerosis. Nat Genet. 2006;38(4):411–413. [DOI] [PubMed] [Google Scholar]
- 192. Smith BN, Topp SD, Fallini C, et al. Mutations in the vesicular trafficking protein annexin A11 are associated with amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(388):eaad9157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Elden AC, Kim HJ, Hart MP, et al. Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature. 2010;466(7310):1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Renton AE, Majounie E, Waite A, et al. ; ITALSGEN Consortium . A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. DeJesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Williams KL, Topp S, Yang S, et al. CCNF mutations in amyotrophic lateral sclerosis and frontotemporal dementia. Nat Commun. 2016;7:11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. van Rheenen W, Shatunov A, Dekker AM, et al. ; PARALS Registry; SLALOM Group; SLAP Registry; FALS Sequencing Consortium; SLAGEN Consortium; NNIPPS Study Group . Genome-wide association analyses identify new risk variants and the genetic architecture of amyotrophic lateral sclerosis. Nat Genet. 2016;48(9):1043–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Bannwarth S, Ait-El-Mkadem S, Chaussenot A, et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain. 2014;137(Pt 8):2329–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Parkinson N, Ince PG, Smith MO, et al. ; MRC Proteomics in ALS Study; FReJA Consortium . ALS phenotypes with mutations in CHMP2B (charged multivesicular body protein 2B). Neurology. 2006;67(6):1074–1077. [DOI] [PubMed] [Google Scholar]
- 200. Mitchell J, Paul P, Chen HJ, et al. Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc Natl Acad Sci U S A. 2010;107(16):7556–7561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Münch C, Sedlmeier R, Meyer T, et al. Point mutations of the p150 subunit of dynactin (DCTN1) gene in ALS. Neurology. 2004;63(4):724–726. [DOI] [PubMed] [Google Scholar]
- 202. Simpson CL, Lemmens R, Miskiewicz K, et al. Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum Mol Genet. 2009;18(3):472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Takahashi Y, Fukuda Y, Yoshimura J, et al. ; JaCALS . ERBB4 mutations that disrupt the neuregulin-ErbB4 pathway cause amyotrophic lateral sclerosis type 19. Am J Hum Genet. 2013;93(5):900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Couthouis J, Hart MP, Erion R, et al. Evaluating the role of the FUS/TLS-related gene EWSR1 in amyotrophic lateral sclerosis. Hum Mol Genet. 2012;21(13):2899–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Chow CY, Landers JE, Bergren SK, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84(1):85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Kwiatkowski TJ Jr, Bosco DA, Leclerc AL, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. [DOI] [PubMed] [Google Scholar]
- 207. Vance C, Rogelj B, Hortobágyi T, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Kaneb HM, Folkmann AW, Belzil VV, et al. Deleterious mutations in the essential mRNA metabolism factor, hGle1, in amyotrophic lateral sclerosis. Hum Mol Genet. 2015;24(5):1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Kim HJ, Kim NC, Wang YD, et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495(7442):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Brenner D, Yilmaz R, Müller K, et al. ; German ALS network MND-NET . Hot-spot KIF5A mutations cause familial ALS. Brain. 2018;141(3):688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Johnson JO, Pioro EP, Boehringer A, et al. ; ITALSGEN . Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat Neurosci. 2014;17(5):664–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Figlewicz DA, Krizus A, Martinoli MG, et al. Variants of the heavy neurofilament subunit are associated with the development of amyotrophic lateral sclerosis. Hum Mol Genet. 1994;3(10):1757–1761. [DOI] [PubMed] [Google Scholar]
- 213. Kenna KP, van Doormaal PT, Dekker AM, et al. ; SLAGEN Consortium . NEK1 variants confer susceptibility to amyotrophic lateral sclerosis. Nat Genet. 2016;48(9):1037–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Maruyama H, Morino H, Ito H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465(7295):223–226. [DOI] [PubMed] [Google Scholar]
- 215. Wu CH, Fallini C, Ticozzi N, et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature. 2012;488(7412):499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Chen YZ, Bennett CL, Huynh HM, et al. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet. 2004;74(6):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Al-Saif A, Al-Mohanna F, Bohlega S. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011;70(6):913–919. [DOI] [PubMed] [Google Scholar]
- 218. Rosen DR, Siddique T, Patterson D, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. [DOI] [PubMed] [Google Scholar]
- 219. Orlacchio A, Babalini C, Borreca A, et al. SPATACSIN mutations cause autosomal recessive juvenile amyotrophic lateral sclerosis. Brain. 2010;133(Pt 2):591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Fecto F, Yan J, Vemula SP, et al. SQSTM1 mutations in familial and sporadic amyotrophic lateral sclerosis. Arch Neurol. 2011;68(11):1440–1446. [DOI] [PubMed] [Google Scholar]
- 221. Couthouis J, Hart MP, Shorter J, et al. A yeast functional screen predicts new candidate ALS disease genes. Proc Natl Acad Sci U S A. 2011;108(52):20881–20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sreedharan J, Blair IP, Tripathi VB, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kabashi E, Valdmanis PN, Dion P, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40(5):572–574. [DOI] [PubMed] [Google Scholar]
- 224. Cirulli ET, Lasseigne BN, Petrovski S, et al. ; FALS Sequencing Consortium . Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347(6229):1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Mackenzie IR, Nicholson AM, Sarkar M, et al. TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics. Neuron. 2017;95(4):808–816.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Rademakers R, van Blitterswijk M. Excess of rare damaging TUBA4A variants suggests cytoskeletal defects in ALS. Neuron. 2014;84(2):241–243. [DOI] [PubMed] [Google Scholar]
- 227. Smith BN, Ticozzi N, Fallini C, et al. ; SLAGEN Consortium . Exome-wide rare variant analysis identifies TUBA4A mutations associated with familial ALS. Neuron. 2014;84(2):324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Deng HX, Chen W, Hong ST, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011;477(7363):211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Nishimura AL, Mitne-Neto M, Silva HC, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75(5):822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Johnson JO, Mandrioli J, Benatar M, et al. ; ITALSGEN Consortium . Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68(5):857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352(6330):77–79. [DOI] [PubMed] [Google Scholar]
- 232. Bendotti C, Marino M, Cheroni C, et al. Dysfunction of constitutive and inducible ubiquitin-proteasome system in amyotrophic lateral sclerosis: implication for protein aggregation and immune response. Prog Neurobiol. 2012;97(2):101–126. [DOI] [PubMed] [Google Scholar]
- 233. Seetharaman SV, Prudencio M, Karch C, Holloway SP, Borchelt DR, Hart PJ. Immature copper-zinc superoxide dismutase and familial amyotrophic lateral sclerosis. Exp Biol Med (Maywood). 2009;234(10):1140–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat Rev Neurosci. 2006;7(9):710–723. [DOI] [PubMed] [Google Scholar]
- 235. Bosco DA, Morfini G, Karabacak NM, et al. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13(11):1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Bosco DA, Landers JE. Genetic determinants of amyotrophic lateral sclerosis as therapeutic targets. CNS Neurol Disord Drug Targets. 2010;9(6):779–790. [DOI] [PubMed] [Google Scholar]
- 237. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. [DOI] [PubMed] [Google Scholar]
- 238. Daoud H, Valdmanis PN, Kabashi E, et al. Contribution of TARDBP mutations to sporadic amyotrophic lateral sclerosis. J Med Genet. 2009;46(2):112–114. [DOI] [PubMed] [Google Scholar]
- 239. Ash PE, Bieniek KF, Gendron TF, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77(4):639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Lashley T, Hardy J, Isaacs AM. RANTing about C9orf72. Neuron. 2013;77(4):597–598. [DOI] [PubMed] [Google Scholar]
- 241. Mori K, Lammich S, Mackenzie IR, et al. hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol. 2013;125(3):413–423. [DOI] [PubMed] [Google Scholar]
- 242. Al-Sarraj S, King A, Troakes C, et al. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122(6):691–702. [DOI] [PubMed] [Google Scholar]
- 243. Boeynaems S, Bogaert E, Kovacs D, et al. Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics. Mol Cell. 2017;65(6):1044–1055.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Freibaum BD, Taylor JP. The role of dipeptide repeats in C9ORF72-related ALS-FTD. Front Mol Neurosci. 2017;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Carra S, Crippa V, Rusmini P, et al. Alteration of protein folding and degradation in motor neuron diseases: implications and protective functions of small heat shock proteins. Prog Neurobiol. 2012;97(2):83–100. [DOI] [PubMed] [Google Scholar]
- 246. Musarò A. State of the art and the dark side of amyotrophic lateral sclerosis. World J Biol Chem. 2010;1(5):62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Dobrowolny G, Aucello M, Rizzuto E, et al. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8(5):425–436. [DOI] [PubMed] [Google Scholar]
- 248. Onesto E, Rusmini P, Crippa V, et al. Muscle cells and motoneurons differentially remove mutant SOD1 causing familial amyotrophic lateral sclerosis. J Neurochem. 2011;118(2):266–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Nagai M, Re DB, Nagata T, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Boillée S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52(1):39–59. [DOI] [PubMed] [Google Scholar]
- 251. Trotti D, Rolfs A, Danbolt NC, Brown RH Jr, Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci. 1999;2(9):848. [DOI] [PubMed] [Google Scholar]
- 252. Philips T, Bento-Abreu A, Nonneman A, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain. 2013;136(Pt 2):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Lobsiger CS, Boillee S, McAlonis-Downes M, et al. Schwann cells expressing dismutase active mutant SOD1 unexpectedly slow disease progression in ALS mice. Proc Natl Acad Sci U S A. 2009;106(11):4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Manjaly ZR, Scott KM, Abhinav K, et al. The sex ratio in amyotrophic lateral sclerosis: a population based study. Amyotroph Lateral Scler. 2010;11(5):439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Philips T, Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10(3):253–263. [DOI] [PubMed] [Google Scholar]
- 256. Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7(11):616–630. [DOI] [PubMed] [Google Scholar]
- 257. Kurtzke JF. Epidemiology of amyotrophic lateral sclerosis. Adv Neurol. 1982;36:281–302. [PubMed] [Google Scholar]
- 258. Haverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118 (Pt 3):707–719. [DOI] [PubMed] [Google Scholar]
- 259. Miller SC, Warnick JE. Protirelin (thyrotropin-releasing hormone) in amyotrophic lateral sclerosis. The role of androgens. Arch Neurol. 1989;46(3):330–335. [DOI] [PubMed] [Google Scholar]
- 260. Ji YX, Zhao M, Liu YL, Chen LS, Hao PL, Sun C. Expression of aromatase and estrogen receptors in lumbar motoneurons of mice. Neurosci Lett. 2017;653:7–11. [DOI] [PubMed] [Google Scholar]
- 261. Trieu VN, Uckun FM. Genistein is neuroprotective in murine models of familial amyotrophic lateral sclerosis and stroke. Biochem Biophys Res Commun. 1999;258(3):685–688. [DOI] [PubMed] [Google Scholar]
- 262. Bruson A, Sambataro F, Querin G, et al. CAG repeat length in androgen receptor gene is not associated with amyotrophic lateral sclerosis. Eur J Neurol. 2012;19(10):1373–1375. [DOI] [PubMed] [Google Scholar]
- 263. Rooney JPK, Visser AE, D’Ovidio F, et al. ; Euro-MOTOR Consortium . A case-control study of hormonal exposures as etiologic factors for ALS in women: Euro-MOTOR. Neurology. 2017;89(12):1283–1290. [DOI] [PubMed] [Google Scholar]
- 264. Trojsi F, Siciliano M, Femiano C, et al. Comparative analysis of C9orf72 and sporadic disease in a large multicenter ALS population: the effect of male sex on survival of C9orf72 positive patients. Front Neurosci. 2019;13:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Suzuki M, Tork C, Shelley B, et al. Sexual dimorphism in disease onset and progression of a rat model of ALS. Amyotroph Lateral Scler. 2007;8(1):20–25. [DOI] [PubMed] [Google Scholar]
- 266. Pfohl SR, Halicek MT, Mitchell CS. Characterization of the contribution of genetic background and gender to disease progression in the SOD1 G93A mouse model of amyotrophic lateral sclerosis: a meta-analysis. J Neuromuscul Dis. 2015;2(2):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Gros-Louis F, Andersen PM, Dupre N, et al. Chromogranin B P413L variant as risk factor and modifier of disease onset for amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2009;106(51):21777–21782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Ricci C, Claudia R, Battistini S, et al. Lack of relationship between the P413L chromogranin B variant and a SALS Italian cohort. Gene. 2015;568(2):186–189. [DOI] [PubMed] [Google Scholar]
- 269. Engelhardt JI, Appel SH. IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol. 1990;47(11):1210–1216. [DOI] [PubMed] [Google Scholar]
- 270. Engelhardt JI, Tajti J, Appel SH. Lymphocytic infiltrates in the spinal cord in amyotrophic lateral sclerosis. Arch Neurol. 1993;50(1):30–36. [DOI] [PubMed] [Google Scholar]
- 271. Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23(3):249–256. [DOI] [PubMed] [Google Scholar]
- 272. Schiffer D, Cordera S, Cavalla P, Migheli A. Reactive astrogliosis of the spinal cord in amyotrophic lateral sclerosis. J Neurol Sci. 1996;139 Suppl:27–33. [DOI] [PubMed] [Google Scholar]
- 273. Nagy D, Kato T, Kushner PD. Reactive astrocytes are widespread in the cortical gray matter of amyotrophic lateral sclerosis. J Neurosci Res. 1994;38(3):336–347. [DOI] [PubMed] [Google Scholar]
- 274. Kushner PD, Stephenson DT, Wright S. Reactive astrogliosis is widespread in the subcortical white matter of amyotrophic lateral sclerosis brain. J Neuropathol Exp Neurol. 1991;50(3):263–277. [DOI] [PubMed] [Google Scholar]
- 275. Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57(7):1282–1289. [DOI] [PubMed] [Google Scholar]
- 276. Levine JB, Kong J, Nadler M, Xu Z. Astrocytes interact intimately with degenerating motor neurons in mouse amyotrophic lateral sclerosis (ALS). Glia. 1999;28(3):215–224. [PubMed] [Google Scholar]
- 277. Gowing G, Philips T, Van Wijmeersch B, et al. Ablation of proliferating microglia does not affect motor neuron degeneration in amyotrophic lateral sclerosis caused by mutant superoxide dismutase. J Neurosci. 2008;28(41):10234–10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Chiu IM, Phatnani H, Kuligowski M, et al. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proc Natl Acad Sci U S A. 2009;106(49):20960–20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Chiu IM, Chen A, Zheng Y, et al. T lymphocytes potentiate endogenous neuroprotective inflammation in a mouse model of ALS. Proc Natl Acad Sci U S A. 2008;105(46):17913–17918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Beers DR, Henkel JS, Zhao W, Wang J, Appel SH. CD4+ T cells support glial neuroprotection, slow disease progression, and modify glial morphology in an animal model of inherited ALS. Proc Natl Acad Sci U S A. 2008;105(40):15558–15563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Brooks BP, Fischbeck KH. Spinal and bulbar muscular atrophy: a trinucleotide-repeat expansion neurodegenerative disease. Trends Neurosci. 1995;18(10):459–461. [DOI] [PubMed] [Google Scholar]
- 282. Fischbeck KH. Kennedy disease. J Inherit Metab Dis. 1997;20(2):152–158. [DOI] [PubMed] [Google Scholar]
- 283. Sobue G, Hashizume Y, Mukai E, Hirayama M, Mitsuma T, Takahashi A. X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain. 1989;112 (Pt 1):209–232. [DOI] [PubMed] [Google Scholar]
- 284. Li M, Sobue G, Doyu M, Mukai E, Hashizume Y, Mitsuma T. Primary sensory neurons in X-linked recessive bulbospinal neuropathy: histopathology and androgen receptor gene expression. Muscle Nerve. 1995;18(3):301–308. [DOI] [PubMed] [Google Scholar]
- 285. Chua JP, Lieberman AP. Pathogenic mechanisms and therapeutic strategies in spinobulbar muscular atrophy. CNS Neurol Disord Drug Targets. 2013;12(8):1146–1156. [PMC free article] [PubMed] [Google Scholar]
- 286. Polo A, Teatini F, D’Anna S, et al. Sensory involvement in X-linked spino-bulbar muscular atrophy (Kennedy’s syndrome): an electrophysiological study. J Neurol. 1996;243(5):388–392. [DOI] [PubMed] [Google Scholar]
- 287. Cortes CJ, Ling SC, Guo LT, et al. Muscle expression of mutant androgen receptor accounts for systemic and motor neuron disease phenotypes in spinal and bulbar muscular atrophy. Neuron. 2014;82(2):295–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Lieberman AP, Yu Z, Murray S, et al. Peripheral androgen receptor gene suppression rescues disease in mouse models of spinal and bulbar muscular atrophy. Cell Rep. 2014;7(3):774–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Rinaldi C, Bott LC, Fischbeck KH. Muscle matters in Kennedy’s disease. Neuron. 2014;82(2):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Rusmini P, Polanco MJ, Cristofani R, et al. Aberrant autophagic response in the muscle of A knock-in mouse model of spinal and bulbar muscular atrophy. Sci Rep. 2015;5:15174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Kazemi-Esfarjani P, Trifiro MA, Pinsky L. Evidence for a repressive function of the long polyglutamine tract in the human androgen receptor: possible pathogenetic relevance for the (CAG)n-expanded neuronopathies. Hum Mol Genet. 1995;4(4):523–527. [DOI] [PubMed] [Google Scholar]
- 292. Belsham DD, Evangelou A, Roy D, Duc VL, Brown TJ. Regulation of gonadotropin-releasing hormone (GnRH) gene expression by 5alpha-dihydrotestosterone in GnRH-secreting GT1-7 hypothalamic neurons. Endocrinology. 1998;139(3):1108–1114. [DOI] [PubMed] [Google Scholar]
- 293. Poletti A, Rampoldi A, Piccioni F, et al. 5Alpha-reductase type 2 and androgen receptor expression in gonadotropin releasing hormone GT1-1 cells. J Neuroendocrinol. 2001;13(4):353–357. [DOI] [PubMed] [Google Scholar]
- 294. Kuhlenbäumer G, Kress W, Ringelstein EB, Stögbauer F. Thirty-seven CAG repeats in the androgen receptor gene in two healthy individuals. J Neurol. 2001;248(1):23–26. [DOI] [PubMed] [Google Scholar]
- 295. Edwards A, Hammond HA, Jin L, Caskey CT, Chakraborty R. Genetic variation at five trimeric and tetrameric tandem repeat loci in four human population groups. Genomics. 1992;12(2):241–253. [DOI] [PubMed] [Google Scholar]
- 296. Madeira JLO, Souza ABC, Cunha FS, et al. A severe phenotype of Kennedy disease associated with a very large CAG repeat expansion. Muscle Nerve. 2018;57(1):E95–E97. [DOI] [PubMed] [Google Scholar]
- 297. La Spada AR, Roling DB, Harding AE, et al. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992;2(4):301–304. [DOI] [PubMed] [Google Scholar]
- 298. Morrison PJ, Mirakhur M, Patterson VH. Discordant repeat size and phenotype in Kennedy syndrome. Clin Genet. 1998;53(4):276–277. [DOI] [PubMed] [Google Scholar]
- 299. Ross CA. Polyglutamine pathogenesis: emergence of unifying mechanisms for Huntington’s disease and related disorders. Neuron. 2002;35(5):819–822. [DOI] [PubMed] [Google Scholar]
- 300. Suzuki K, Katsuno M, Banno H, et al. CAG repeat size correlates to electrophysiological motor and sensory phenotypes in SBMA. Brain. 2008;131(Pt 1):229–239. [DOI] [PubMed] [Google Scholar]
- 301. Adachi H, Katsuno M, Minamiyama M, et al. Widespread nuclear and cytoplasmic accumulation of mutant androgen receptor in SBMA patients. Brain. 2005;128(Pt 3):659–670. [DOI] [PubMed] [Google Scholar]
- 302. Rhodes LE, Freeman BK, Auh S, et al. Clinical features of spinal and bulbar muscular atrophy. Brain. 2009;132(Pt 12):3242–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Johansen JA, Yu Z, Mo K, et al. Recovery of function in a myogenic mouse model of spinal bulbar muscular atrophy. Neurobiol Dis. 2009;34(1):113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Halievski K, Kemp MQ, Breedlove SM, Miller KE, Jordan CL. Non-cell-autonomous regulation of retrograde motoneuronal axonal transport in an SBMA mouse model. eNeuro. 2016;3(4):e0062–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Poort JE, Rheuben MB, Breedlove SM, Jordan CL. Neuromuscular junctions are pathological but not denervated in two mouse models of spinal bulbar muscular atrophy. Hum Mol Genet. 2016;25(17):3768–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Ruigrok AN, Salimi-Khorshidi G, Lai MC, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Rushton JP, Ankney CD. Whole brain size and general mental ability: a review. Int J Neurosci. 2009;119(5):691–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Lüders E, Steinmetz H, Jäncke L. Brain size and grey matter volume in the healthy human brain. Neuroreport. 2002;13(17):2371–2374. [DOI] [PubMed] [Google Scholar]
- 309. Allen JS, Damasio H, Grabowski TJ. Normal neuroanatomical variation in the human brain: an MRI-volumetric study. Am J Phys Anthropol. 2002;118(4):341–358. [DOI] [PubMed] [Google Scholar]
- 310. Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007;62(8):847–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Kornstein SG, Schatzberg AF, Thase ME, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157(9):1445–1452. [DOI] [PubMed] [Google Scholar]
- 312. Rasgon NL, Dunkin J, Fairbanks L, et al. Estrogen and response to sertraline in postmenopausal women with major depressive disorder: a pilot study. J Psychiatr Res. 2007;41(3-4):338–343. [DOI] [PubMed] [Google Scholar]
- 313. Parsey RV, Oquendo MA, Simpson NR, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954(2):173–182. [DOI] [PubMed] [Google Scholar]
- 314. Haier RJ, Jung RE, Yeo RA, Head K, Alkire MT. The neuroanatomy of general intelligence: sex matters. Neuroimage. 2005;25(1):320–327. [DOI] [PubMed] [Google Scholar]
- 315. Smith ES, Junger J, Derntl B, Habel U. The transsexual brain–a review of findings on the neural basis of transsexualism. Neurosci Biobehav Rev. 2015;59:251–266. [DOI] [PubMed] [Google Scholar]
- 316. Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138(3):921–928. [DOI] [PubMed] [Google Scholar]
- 317. Hutchison JB, Beyer C, Hutchison RE, Wozniak A. Sex differences in the regulation of embryonic brain aromatase. J Steroid Biochem Mol Biol. 1997;61(3-6):315–322. [DOI] [PubMed] [Google Scholar]
- 318. Fuente-Martin E, Garcia-Caceres C, Morselli E, et al. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev Endocr Metab Disord. 2013;14(4):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Della Torre S, Benedusi V, Fontana R, Maggi A. Energy metabolism and fertility: a balance preserved for female health. Nat Rev Endocrinol. 2014;10(1):13–23. [DOI] [PubMed] [Google Scholar]
- 320. McCarthy MM. Sexual differentiation of the brain in man and animals: of relevance to Klinefelter syndrome? Am J Med Genet C Semin Med Genet. 2013;163C(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Collado P, Beyer C, Hutchison JB, Holman SD. Hypothalamic distribution of astrocytes is gender-related in Mongolian gerbils. Neurosci Lett. 1995;184(2):86–89. [DOI] [PubMed] [Google Scholar]
- 322. Garcia-Segura LM, Suarez I, Segovia S, et al. The distribution of glial fibrillary acidic protein in the adult rat brain is influenced by the neonatal levels of sex steroids. Brain Res. 1988;456(2):357–363. [DOI] [PubMed] [Google Scholar]
- 323. Suárez I, Bodega G, Rubio M, Fernandez B. Sexual dimorphism in the distribution of glial fibrillary acidic protein in the supraoptic nucleus of the hamster. J Anat. 1991;178:79–82. [PMC free article] [PubMed] [Google Scholar]
- 324. Amateau SK, McCarthy MM. Sexual differentiation of astrocyte morphology in the developing rat preoptic area. J Neuroendocrinol. 2002;14(11):904–910. [DOI] [PubMed] [Google Scholar]
- 325. Amateau SK, McCarthy MM. Induction of PGE2 by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7(6):643–650. [DOI] [PubMed] [Google Scholar]
- 326. Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol. 2008;511(5):599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Garcia-Segura LM, Dueñas M, Busiguina S, Naftolin F, Chowen JA. Gonadal hormone regulation of neuronal-glial interactions in the developing neuroendocrine hypothalamus. J Steroid Biochem Mol Biol. 1995;53(1-6):293–298. [DOI] [PubMed] [Google Scholar]
- 328. Kuo J, Hamid N, Bondar G, Dewing P, Clarkson J, Micevych P. Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biol Sex Differ. 2010;1(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40(4):602–619. [DOI] [PubMed] [Google Scholar]
- 330. Suárez I, Bodega G, Rubio M, Fernández B. Sexual dimorphism in the hamster cerebellum demonstrated by glial fibrillary acidic protein (GFAP) and vimentin immunoreactivity. Glia. 1992;5(1):10–16. [DOI] [PubMed] [Google Scholar]
- 331. Crain JM, Nikodemova M, Watters JJ. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J Neurosci Res. 2013;91(9):1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Crain JM, Watters JJ. Microglial P2 purinergic receptor and immunomodulatory gene transcripts vary by region, sex, and age in the healthy mouse CNS. Transcr Open Access. 2015;3(2):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Villa A, Gelosa P, Castiglioni L, et al. Sex-specific features of microglia from adult mice. Cell Rep. 2018;23(12):3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Poletti A. The polyglutamine tract of androgen receptor: from functions to dysfunctions in motor neurons. Front Neuroendocrinol. 2004;25(1):1–26. [DOI] [PubMed] [Google Scholar]
- 335. King W, Greene G. Estrogen receptor localization by use of monoclonal receptor antibodies. Nature. 1984;307:745–747. [DOI] [PubMed] [Google Scholar]
- 336. Faus H, Haendler B. Post-translational modifications of steroid receptors. Biomed Pharmacother. 2006;60(9):520–528. [DOI] [PubMed] [Google Scholar]
- 337. Maggi A. Liganded and unliganded activation of estrogen receptor and hormone replacement therapies. Biochim Biophys Acta. 2011;1812(8):1054–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. [DOI] [PubMed] [Google Scholar]
- 339. Chung WC, Pak TR, Suzuki S, Pouliot WA, Andersen ME, Handa RJ. Detection and localization of an estrogen receptor beta splice variant protein (ERbeta2) in the adult female rat forebrain and midbrain regions. J Comp Neurol. 2007;505(3):249–267. [DOI] [PubMed] [Google Scholar]
- 340. Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19(8):1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Jacome LF, Gautreaux C, Inagaki T, et al. Estradiol and ERβ agonists enhance recognition memory, and DPN, an ERβ agonist, alters brain monoamines. Neurobiol Learn Mem. 2010;94(4):488–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Ishunina TA, Kruijver FP, Balesar R, Swaab DF. Differential expression of estrogen receptor alpha and beta immunoreactivity in the human supraoptic nucleus in relation to sex and aging. J Clin Endocrinol Metab. 2000;85(9):3283–3291. [DOI] [PubMed] [Google Scholar]
- 344. Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 2001;64(3):251–267. [DOI] [PubMed] [Google Scholar]
- 345. Santagati S, Melcangi RC, Celotti F, Martini L, Maggi A. Estrogen receptor is expressed in different types of glial cells in culture. J Neurochem. 1994;63(6):2058–2064. [DOI] [PubMed] [Google Scholar]
- 346. Vegeto E, Bonincontro C, Pollio G, et al. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J Neurosci. 2001;21(6):1809–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Brown TJ, Naftolin F, Maclusky NJ. Sex differences in estrogen receptor binding in the rat hypothalamus: effects of subsaturating pulses of estradiol. Brain Res. 1992;578(1-2):129–134. [DOI] [PubMed] [Google Scholar]
- 348. Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol. 2002;454(2):115–139. [DOI] [PubMed] [Google Scholar]
- 349. Loyd DR, Murphy AZ. Androgen and estrogen (alpha) receptor localization on periaqueductal gray neurons projecting to the rostral ventromedial medulla in the male and female rat. J Chem Neuroanat. 2008;36(3-4):216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Kelly DA, Varnum MM, Krentzel AA, Krug S, Forger NG. Differential control of sex differences in estrogen receptor α in the bed nucleus of the stria terminalis and anteroventral periventricular nucleus. Endocrinology. 2013;154(10):3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology. 2005;81(6):391–399. [DOI] [PubMed] [Google Scholar]
- 352. Ciana P, Raviscioni M, Mussi P, et al. In vivo imaging of transcriptionally active estrogen receptors. Nat Med. 2003;9(1):82–86. [DOI] [PubMed] [Google Scholar]
- 353. Stell A, Belcredito S, Ciana P, Maggi A. Molecular imaging provides novel insights on estrogen receptor activity in mouse brain. Mol Imaging. 2008;7(6):283–292. [PMC free article] [PubMed] [Google Scholar]
- 354. Guerini V, Sau D, Scaccianoce E, et al. The androgen derivative 5alpha-androstane-3beta,17beta-diol inhibits prostate cancer cell migration through activation of the estrogen receptor beta subtype. Cancer Res. 2005;65(12):5445–5453. [DOI] [PubMed] [Google Scholar]
- 355. Petersen SL, Krishnan S, Aggison LK, Intlekofer KA, Moura PJ. Sexual differentiation of the gonadotropin surge release mechanism: a new role for the canonical NfκB signaling pathway. Front Neuroendocrinol. 2012;33(1):36–44. [DOI] [PubMed] [Google Scholar]
- 356. Fester L, Prange-Kiel J, Zhou L, et al. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol. 2012;131(1-2):24–29. [DOI] [PubMed] [Google Scholar]
- 357. Al Sweidi S, Sánchez MG, Bourque M, Morissette M, Dluzen D, Di Paolo T. Oestrogen receptors and signalling pathways: implications for neuroprotective effects of sex steroids in Parkinson’s disease. J Neuroendocrinol. 2012;24(1):48–61. [DOI] [PubMed] [Google Scholar]
- 358. Joseph EK, Parada CA, Levine JD. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105(1-2):143–150. [DOI] [PubMed] [Google Scholar]
- 359. Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202(2):223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Brailoiu E, Dun SL, Brailoiu GC, et al. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311–321. [DOI] [PubMed] [Google Scholar]
- 361. Hammond R, Gibbs RB. GPR30 is positioned to mediate estrogen effects on basal forebrain cholinergic neurons and cognitive performance. Brain Res. 2011;1379:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Lee E, Sidoryk-Wêgrzynowicz M, Wang N, et al. GPR30 regulates glutamate transporter GLT-1 expression in rat primary astrocytes. J Biol Chem. 2012;287(32):26817–26828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Kastner P, Bocquel MT, Turcotte B, et al. Transient expression of human and chicken progesterone receptors does not support alternative translational initiation from a single mRNA as the mechanism generating two receptor isoforms. J Biol Chem. 1990;265(21):12163–12167. [PubMed] [Google Scholar]
- 364. Conneely OM, Maxwell BL, Toft DO, Schrader WT, O’Malley BW. The A and B forms of the chicken progesterone receptor arise by alternate initiation of translation of a unique mRNA. Biochem Biophys Res Commun. 1987;149(2):493–501. [DOI] [PubMed] [Google Scholar]
- 365. Cork DM, Lennard TW, Tyson-Capper AJ. Alternative splicing and the progesterone receptor in breast cancer. Breast Cancer Res. 2008;10(3):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Hirata S, Shoda T, Kato J, Hoshi K. Novel isoforms of the mRNA for human female sex steroid hormone receptors. J Steroid Biochem Mol Biol. 2002;83(1-5):25–30. [DOI] [PubMed] [Google Scholar]
- 367. Kato J, Hirata S, Nozawa A, Yamada-Mouri N. Gene expression of progesterone receptor isoforms in the rat brain. Horm Behav. 1994;28(4):454–463. [DOI] [PubMed] [Google Scholar]
- 368. Guerra-Araiza C, Cerbón MA, Morimoto S, Camacho-Arroyo I. Progesterone receptor isoforms expression pattern in the rat brain during the estrous cycle. Life Sci. 2000;66(18):1743–1752. [DOI] [PubMed] [Google Scholar]
- 369. Guerra-Araiza C, Coyoy-Salgado A, Camacho-Arroyo I. Sex differences in the regulation of progesterone receptor isoforms expression in the rat brain. Brain Res Bull. 2002;59(2):105–109. [DOI] [PubMed] [Google Scholar]
- 370. Guerra-Araiza C, Villamar-Cruz O, González-Arenas A, Chavira R, Camacho-Arroyo I. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15(10):984–990. [DOI] [PubMed] [Google Scholar]
- 371. Blaustein JD, Ryer HI, Feder HH. A sex difference in the progestin receptor system of guinea pig brain. Neuroendocrinology. 1980;31(6):403–409. [DOI] [PubMed] [Google Scholar]
- 372. Samama B, Aron C. Induction of progestin receptors in the mediobasal hypothalamus of gonadally intact male rats primed with estrogen in relation to display of lordosis behavior. J Steroid Biochem Mol Biol. 1991;39(2):215–220. [DOI] [PubMed] [Google Scholar]
- 373. Rodriguez-Sierra JF, Hagley MT, Hendricks SE. Anxiolytic effects of progesterone are sexually dimorphic. Life Sci. 1986;38(20):1841–1845. [DOI] [PubMed] [Google Scholar]
- 374. Dufourny L, Warembourg M. Colocalization of progesterone receptor and somatostatin immunoreactivities in the hypothalamus of the male and female guinea pig. Neuroendocrinology. 1996;64(3):215–224. [DOI] [PubMed] [Google Scholar]
- 375. Coirini H, Johnson AE, Schumacher M, McEwen BS. Sex differences in the regulation of oxytocin receptors by ovarian steroids in the ventromedial hypothalamus of the rat. Neuroendocrinology. 1992;55(3):269–275. [DOI] [PubMed] [Google Scholar]
- 376. Romano GJ, Krust A, Pfaff DW. Expression and estrogen regulation of progesterone receptor mRNA in neurons of the mediobasal hypothalamus: an in situ hybridization study. Mol Endocrinol. 1989;3(8):1295–1300. [DOI] [PubMed] [Google Scholar]
- 377. Jung-Testas I, Baulieu EE. Steroid hormone receptors and steroid action in rat glial cells of the central and peripheral nervous system. J Steroid Biochem Mol Biol. 1998;65(1-6):243–251. [DOI] [PubMed] [Google Scholar]
- 378. Bali N, Morgan TE, Finch CE. Pgrmc1: new roles in the microglial mediation of progesterone-antagonism of estradiol-dependent neurite sprouting and in microglial activation. Front Neurosci. 2013;7:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K. Steroid hormone receptor expression and function in microglia. Glia. 2008;56(6):659–674. [DOI] [PubMed] [Google Scholar]
- 380. Acconcia F, Ascenzi P, Bocedi A, et al. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16(1):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Pedram A, Razandi M, Deschenes RJ, Levin ER. DHHC-7 and -21 are palmitoylacyltransferases for sex steroid receptors. Mol Biol Cell. 2012;23(1):188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100(5):2231–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Meffre D, Labombarda F, Delespierre B, et al. Distribution of membrane progesterone receptor alpha in the male mouse and rat brain and its regulation after traumatic brain injury. Neuroscience. 2013;231:111–124. [DOI] [PubMed] [Google Scholar]
- 384. Pfaff DW, Kow LM, Loose MD, Flanagan-Cato LM. Reverse engineering the lordosis behavior circuit. Horm Behav. 2008;54(3):347–354. [DOI] [PubMed] [Google Scholar]
- 385. Pollio G, Xue P, Zanisi M, Nicolin A, Maggi A. Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Brain Res Mol Brain Res. 1993;19(1-2):135–139. [DOI] [PubMed] [Google Scholar]
- 386. Mani SK, Allen JM, Clark JH, Blaustein JD, O’Malley BW. Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science. 1994;265(5176):1246–1249. [DOI] [PubMed] [Google Scholar]
- 387. Camacho-Arroyo I, Pasapera AM, Pérez-Palacios G, Cerbón MA. Progesterone and its metabolites in central nervous system function. Rev Invest Clin. 1995;47(4):329–340. [PubMed] [Google Scholar]
- 388. Levine JE, Chappell PE, Schneider JS, Sleiter NC, Szabo M. Progesterone receptors as neuroendocrine integrators. Front Neuroendocrinol. 2001;22(2):69–106. [DOI] [PubMed] [Google Scholar]
- 389. Brinton RD, Thompson RF, Foy et al. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29(2):313–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390. Lubahn DB, Joseph DR, Sullivan PM, Willard HF, French FS, Wilson EM. Cloning of human androgen receptor complementary DNA and localization to the X chromosome. Science. 1988;240(4850):327–330. [DOI] [PubMed] [Google Scholar]
- 391. Matsumoto T, Sakari M, Okada M, et al. The androgen receptor in health and disease. Annu Rev Physiol. 2013;75:201–224. [DOI] [PubMed] [Google Scholar]
- 392. Jenster G, Trapman J, Brinkmann AO. Nuclear import of the human androgen receptor. Biochem J. 1993;293(Pt 3):761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Jenster G, van der Korput HA, Trapman J, Brinkmann AO. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem. 1995;270(13):7341–7346. [DOI] [PubMed] [Google Scholar]
- 394. Spencer TE, Jenster G, Burcin MM, et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389(6647):194–198. [DOI] [PubMed] [Google Scholar]
- 395. Tyagi RK, Lavrovsky Y, Ahn SC, Song CS, Chatterjee B, Roy AK. Dynamics of intracellular movement and nucleocytoplasmic recycling of the ligand-activated androgen receptor in living cells. Mol Endocrinol. 2000;14(8):1162–1174. [DOI] [PubMed] [Google Scholar]
- 396. Kruijver FP, Fernández-Guasti A, Fodor M, Kraan EM, Swaab DF. Sex differences in androgen receptors of the human mamillary bodies are related to endocrine status rather than to sexual orientation or transsexuality. J Clin Endocrinol Metab. 2001;86(2):818–827. [DOI] [PubMed] [Google Scholar]
- 397. Vismara G, Simonini F, Onesto E, et al. Androgens inhibit androgen receptor promoter activation in motor neurons. Neurobiol Dis. 2009;33(3):395–404. [DOI] [PubMed] [Google Scholar]
- 398. Swaab DF, Chung WC, Kruijver FP, Hofman MA, Ishunina TA. Structural and functional sex differences in the human hypothalamus. Horm Behav. 2001;40(2):93–98. [DOI] [PubMed] [Google Scholar]
- 399. Fernández-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425(3):422–435. [DOI] [PubMed] [Google Scholar]
- 400. Poletti A, Melcangi RC, Negri-Cesi P, Maggi R, Martini L. Steroid binding and metabolism in the luteinizing hormone-releasing hormone-producing neuronal cell line GT1-1. Endocrinology. 1994;135(6):2623–2628. [DOI] [PubMed] [Google Scholar]
- 401. Beyenburg S, Watzka M, Clusmann H, et al. Androgen receptor mRNA expression in the human hippocampus. Neurosci Lett. 2000;294(1):25–28. [DOI] [PubMed] [Google Scholar]
- 402. Lumbroso S, Sandillon F, Georget V, et al. Immunohistochemical localization and immunoblotting of androgen receptor in spinal neurons of male and female rats. Eur J Endocrinol. 1996;134(5):626–632. [DOI] [PubMed] [Google Scholar]
- 403. Fargo KN, Galbiati M, Foecking EM, Poletti A, Jones KJ. Androgen regulation of axon growth and neurite extension in motoneurons. Horm Behav. 2008;53(5):716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404. Yu WH, McGinnis MY. Androgen receptors in cranial nerve motor nuclei of male and female rats. J Neurobiol. 2001;46(1):1–10. [DOI] [PubMed] [Google Scholar]
- 405. Kondo N, Katsuno M, Adachi H, et al. Heat shock factor-1 influences pathological lesion distribution of polyglutamine-induced neurodegeneration. Nat Commun. 2013;4:1405. [DOI] [PubMed] [Google Scholar]
- 406. Palazzolo I, Gliozzi A, Rusmini P, et al. The role of the polyglutamine tract in androgen receptor. J Steroid Biochem Mol Biol. 2008;108(3-5):245–253. [DOI] [PubMed] [Google Scholar]
- 407. Pozzi P, Bendotti C, Simeoni S, et al. Androgen 5-alpha-reductase type 2 is highly expressed and active in rat spinal cord motor neurones. J Neuroendocrinol. 2003;15(9):882–887. [DOI] [PubMed] [Google Scholar]
- 408. Marron TU, Guerini V, Rusmini P, et al. Androgen-induced neurite outgrowth is mediated by neuritin in motor neurones. J Neurochem. 2005;92(1):10–20. [DOI] [PubMed] [Google Scholar]
- 409. Fargo KN, Alexander TD, Tanzer L, Poletti A, Jones KJ. Androgen regulates neuritin mRNA levels in an in vivo model of steroid-enhanced peripheral nerve regeneration. J Neurotrauma. 2008;25(5):561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410. Jo DH, Abdallah MA, Young J, Baulieu EE, Robel P. Pregnenolone, dehydroepiandrosterone, and their sulfate and fatty acid esters in the rat brain. Steroids. 1989;54(3):287–297. [DOI] [PubMed] [Google Scholar]
- 411. Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM. Glia-neuron crosstalk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev. 2005;48(2):273–286. [DOI] [PubMed] [Google Scholar]
- 412. Schumacher M, Weill-Engerer S, Liere P, et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71(1):3–29. [DOI] [PubMed] [Google Scholar]
- 413. Jurevics H, Morell P. Cholesterol for synthesis of myelin is made locally, not imported into brain. J Neurochem. 1995;64(2):895–901. [DOI] [PubMed] [Google Scholar]
- 414. Midzak A, Rone M, Aghazadeh Y, Culty M, Papadopoulos V. Mitochondrial protein import and the genesis of steroidogenic mitochondria. Mol Cell Endocrinol. 2011;336(1-2):70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Kuhlmann AC, Guilarte TR. Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem. 2000;74(4):1694–1704. [DOI] [PubMed] [Google Scholar]
- 416. Maeda J, Higuchi M, Inaji M, et al. Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res. 2007;1157:100–111. [DOI] [PubMed] [Google Scholar]
- 417. Cosenza-Nashat M, Zhao ML, Suh HS, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Gottfried-Blackmore A, Sierra A, Jellinck PH, McEwen BS, Bulloch K. Brain microglia express steroid-converting enzymes in the mouse. J Steroid Biochem Mol Biol. 2008;109(1-2):96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Choi J, Ifuku M, Noda M, Guilarte TR. Translocator protein (18 kDa)/peripheral benzodiazepine receptor specific ligands induce microglia functions consistent with an activated state. Glia. 2011;59(2):219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420. Girard C, Liu S, Adams D, et al. Axonal regeneration and neuroinflammation: roles for the translocator protein 18 kDa. J Neuroendocrinol. 2012;24(1):71–81. [DOI] [PubMed] [Google Scholar]
- 421. Rapic S, Backes H, Viel T, et al. Imaging microglial activation and glucose consumption in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34(1):351–354. [DOI] [PubMed] [Google Scholar]
- 422. Watzka M, Bidlingmaier F, Schramm J, Klingmüller D, Stoffel-Wagner B. Sex- and age-specific differences in human brain CYP11A1 mRNA expression. J Neuroendocrinol. 1999;11(12):901–905. [DOI] [PubMed] [Google Scholar]
- 423. Lavaque E, Mayen A, Azcoitia I, Tena-Sempere M, Garcia-Segura LM. Sex differences, developmental changes, response to injury and cAMP regulation of the mRNA levels of steroidogenic acute regulatory protein, cytochrome p450scc, and aromatase in the olivocerebellar system. J Neurobiol. 2006;66(3):308–318. [DOI] [PubMed] [Google Scholar]
- 424. Le Goascogne C, Robel P, Gouézou M, Sananès N, Baulieu EE, Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987;237(4819):1212–1215. [DOI] [PubMed] [Google Scholar]
- 425. Strömstedt M, Waterman MR. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res Mol Brain Res. 1995;34(1):75–88. [DOI] [PubMed] [Google Scholar]
- 426. Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. Expression of the steroidogenic enzyme P450scc in the central and peripheral nervous systems during rodent embryogenesis. Endocrinology. 1995;136(6):2689–2696. [DOI] [PubMed] [Google Scholar]
- 427. Kimoto T, Tsurugizawa T, Ohta Y, et al. Neurosteroid synthesis by cytochrome p450-containing systems localized in the rat brain hippocampal neurons: N-methyl-D-aspartate and calcium-dependent synthesis. Endocrinology. 2001;142(8):3578–3589. [DOI] [PubMed] [Google Scholar]
- 428. Mellon SH, Deschepper CF. Neurosteroid biosynthesis: genes for adrenal steroidogenic enzymes are expressed in the brain. Brain Res. 1993;629(2):283–292. [DOI] [PubMed] [Google Scholar]
- 429. Hawrylycz MJ, Lein ES, Guillozet-Bongaarts et al. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012;489(7416):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430. Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25(6):947–970. [DOI] [PubMed] [Google Scholar]
- 431. Luu The V, Lachance Y, Labrie C, et al. Full length cDNA structure and deduced amino acid sequence of human 3 beta-hydroxy-5-ene steroid dehydrogenase. Mol Endocrinol. 1989;3(8):1310–1312. [DOI] [PubMed] [Google Scholar]
- 432. Rhéaume E, Lachance Y, Zhao HF, et al. Structure and expression of a new complementary DNA encoding the almost exclusive 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase in human adrenals and gonads. Mol Endocrinol. 1991;5(8):1147–1157. [DOI] [PubMed] [Google Scholar]
- 433. Inoue T, Akahira J, Suzuki T, et al. Progesterone production and actions in the human central nervous system and neurogenic tumors. J Clin Endocrinol Metab. 2002;87(11):5325–5331. [DOI] [PubMed] [Google Scholar]
- 434. Dupont E, Simard J, Luu-The V, Labrie F, Pelletier G. Localization of 3 beta-hydroxysteroid dehydrogenase in rat brain as studied by in situ hybridization. Mol Cell Neurosci. 1994;5(2):119–123. [DOI] [PubMed] [Google Scholar]
- 435. Guennoun R, Fiddes RJ, Gouézou M, Lombès M, Baulieu EE. A key enzyme in the biosynthesis of neurosteroids, 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4-isomerase (3 beta-HSD), is expressed in rat brain. Brain Res Mol Brain Res. 1995;30(2):287–300. [DOI] [PubMed] [Google Scholar]
- 436. Jung-Testas I, Hu ZY, Baulieu EE, Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology. 1989;125(4):2083–2091. [DOI] [PubMed] [Google Scholar]
- 437. Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140(8):3843–3852. [DOI] [PubMed] [Google Scholar]
- 438. Guennoun R, Schumacher M, Robert F, et al. Neurosteroids: expression of functional 3beta-hydroxysteroid dehydrogenase by rat sensory neurons and Schwann cells. Eur J Neurosci. 1997;9(11):2236–2247. [DOI] [PubMed] [Google Scholar]
- 439. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440. Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273(6):3158–3165. [DOI] [PubMed] [Google Scholar]
- 441. Fevold HR, Lorence MC, McCarthy JL, et al. Rat P450(17 alpha) from testis: characterization of a full-length cDNA encoding a unique steroid hydroxylase capable of catalyzing both delta 4- and delta 5-steroid-17,20-lyase reactions. Mol Endocrinol. 1989;3(6):968–975. [DOI] [PubMed] [Google Scholar]
- 442. Flück CE, Miller WL, Auchus RJ. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J Clin Endocrinol Metab. 2003;88(8):3762–3766. [DOI] [PubMed] [Google Scholar]
- 443. Kohchi C, Ukena K, Tsutsui K. Age- and region-specific expressions of the messenger RNAs encoding for steroidogenic enzymes p450scc, P450c17 and 3beta-HSD in the postnatal rat brain. Brain Res. 1998;801(1-2):233–238. [DOI] [PubMed] [Google Scholar]
- 444. Shibuya K, Takata N, Hojo Y, et al. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim Biophys Acta. 2003;1619(3):301–316. [DOI] [PubMed] [Google Scholar]
- 445. Marchais-Oberwinkler S, Henn C, Möller G, et al. 17β-Hydroxysteroid dehydrogenases (17β-HSDs) as therapeutic targets: protein structures, functions, and recent progress in inhibitor development. J Steroid Biochem Mol Biol. 2011;125(1-2):66–82. [DOI] [PubMed] [Google Scholar]
- 446. Reddy VV. Estrogen metabolism in neural tissues of rabbits: 17 beta - hydroxysteroid oxidoreductase activity. Steroids. 1979;34(2):207–215. [DOI] [PubMed] [Google Scholar]
- 447. Resko JA, Stadelman HL, Norman RL. 17 beta-hydroxysteroid dehydrogenase activity in the pituitary gland and neural tissue of Rhesus monkeys. J Steroid Biochem. 1979;11(4):1429–1434. [DOI] [PubMed] [Google Scholar]
- 448. Pelletier G, Luu-The V, Labrie F. Immunocytochemical localization of type I 17 beta-hydroxysteroid dehydrogenase in the rat brain. Brain Res. 1995;704(2):233–239. [DOI] [PubMed] [Google Scholar]
- 449. Mensah-Nyagan AM, Feuilloley M, Do-Rego JL, et al. Localization of 17beta-hydroxysteroid dehydrogenase and characterization of testosterone in the brain of the male frog. Proc Natl Acad Sci U S A. 1996;93(4):1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450. Mensah-Nyagan AG, Do-Rego JL, Feuilloley M, et al. In vivo and in vitro evidence for the biosynthesis of testosterone in the telencephalon of the female frog. J Neurochem. 1996;67(1):413–422. [DOI] [PubMed] [Google Scholar]
- 451. Beyenburg S, Watzka M, Blümcke I, et al. Expression of mRNAs encoding for 17beta-hydroxisteroid dehydrogenase isozymes 1, 2, 3 and 4 in epileptic human hippocampus. Epilepsy Res. 2000;41(1):83–91. [DOI] [PubMed] [Google Scholar]
- 452. Stoffel-Wagner B, Watzka M, Steckelbroeck S, Schramm J, Bidlingmaier JF, Klingmüller D. Expression of 17beta-hydroxysteroid dehydrogenase types 1, 2, 3 and 4 in the human temporal lobe. J Endocrinol. 1999;160(1):119–126. [DOI] [PubMed] [Google Scholar]
- 453. Steckelbroeck S, Watzka M, Reissinger A, et al. Characterisation of estrogenic 17beta-hydroxysteroid dehydrogenase (17beta-HSD) activity in the human brain. J Steroid Biochem Mol Biol. 2003;86(1):79–92. [DOI] [PubMed] [Google Scholar]
- 454. Yan SD, Fu J, Soto C, et al. An intracellular protein that binds amyloid-beta peptide and mediates neurotoxicity in Alzheimer’s disease. Nature. 1997;389(6652):689–695. [DOI] [PubMed] [Google Scholar]
- 455. Yang SY, He XY, Miller D. HSD17B10: a gene involved in cognitive function through metabolism of isoleucine and neuroactive steroids. Mol Genet Metab. 2007;92(1-2):36–42. [DOI] [PubMed] [Google Scholar]
- 456. Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with gamma-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270(3):1223–1229. [PubMed] [Google Scholar]
- 457. Russell DW, Wilson JD. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. [DOI] [PubMed] [Google Scholar]
- 458. Negri-Cesi P, Poletti A, Celotti F. Metabolism of steroids in the brain: a new insight into the role of 5alpha-reductase and aromatase in brain differentiation and functions. J Steroid Biochem Mol Biol. 1996;58(5-6):455–466. [DOI] [PubMed] [Google Scholar]
- 459. Poletti A, Celotti F, Rumio C, Rabuffetti M, Martini L. Identification of type 1 5alpha-reductase in myelin membranes of male and female rat brain. Mol Cell Endocrinol. 1997;129(2):181–190. [DOI] [PubMed] [Google Scholar]
- 460. Colciago A, Celotti F, Pravettoni A, Mornati O, Martini L, Negri-Cesi P. Dimorphic expression of testosterone metabolizing enzymes in the hypothalamic area of developing rats. Brain Res Dev Brain Res. 2005;155(2):107–116. [DOI] [PubMed] [Google Scholar]
- 461. Celotti F, Negri-Cesi P, Poletti A. Steroid metabolism in the mammalian brain: 5alpha-reduction and aromatization. Brain Res Bull. 1997;44(4):365–375. [DOI] [PubMed] [Google Scholar]
- 462. Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol. 2003;86(3-5):219–224. [DOI] [PubMed] [Google Scholar]
- 463. Pareto D, Biegon A, Alexoff D, et al. In vivo imaging of brain aromatase in female baboons: [11C]vorozole kinetics and effect of the menstrual cycle. Mol Imaging. 2013;12(8):518–524. [DOI] [PubMed] [Google Scholar]
- 464. Zuloaga KL, Davis CM, Zhang W, Alkayed NJ. Role of aromatase in sex-specific cerebrovascular endothelial function in mice. Am J Physiol Heart Circ Physiol. 2014;306(7):H929–H937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465. Di Nardo G, Gilardi G. Human aromatase: perspectives in biochemistry and biotechnology. Biotechnol Appl Biochem. 2013;60(1):92–101. [DOI] [PubMed] [Google Scholar]
- 466. Garcia-Segura LM, Veiga S, Sierra A, Melcangi RC, Azcoitia I. Aromatase: a neuroprotective enzyme. Prog Neurobiol. 2003;71(1):31–41. [DOI] [PubMed] [Google Scholar]
- 467. Choi CI, Lee YD, Gwag BJ, Cho SI, Kim SS, Suh-Kim H. Effects of estrogen on lifespan and motor functions in female hSOD1 G93A transgenic mice. J Neurol Sci. 2008;268(1-2):40–47. [DOI] [PubMed] [Google Scholar]
- 468. Groeneveld GJ, Van Muiswinkel FL, Sturkenboom JM, Wokke JH, Bär PR, Van den Berg LH. Ovariectomy and 17beta-estradiol modulate disease progression of a mouse model of ALS. Brain Res. 2004;1021(1):128–131. [DOI] [PubMed] [Google Scholar]
- 469. Heitzer M, Kaiser S, Kanagaratnam M, Zendedel A, Hartmann P, Beyer C, Johann S. Administration of 17β-estradiol improves motoneuron survival and down-regulates inflammasome activation in male SOD1(G93A) ALS mice. Mol Neurobiol. 2017;54(10):8429–8443. [DOI] [PubMed] [Google Scholar]
- 470. Meda C, Vegeto E, Pollio G, et al. Oestrogen prevention of neural cell death correlates with decreased expression of mRNA for the pro-apoptotic protein nip-2. J Neuroendocrinol. 2000;12(11):1051–1059. [DOI] [PubMed] [Google Scholar]
- 471. Singer CA, McMillan PJ, Dobie DJ, Dorsa DM. Effects of estrogen replacement on choline acetyltransferase and trkA mRNA expression in the basal forebrain of aged rats. Brain Res. 1998;789(2):343–346. [DOI] [PubMed] [Google Scholar]
- 472. Grimm A, Schmitt K, Lang UE, Mensah-Nyagan AG, Eckert A. Improvement of neuronal bioenergetics by neurosteroids: implications for age-related neurodegenerative disorders. Biochim Biophys Acta. 2014;1842(12 Pt A):2427–2438. [DOI] [PubMed] [Google Scholar]
- 473. Hara Y, Yuk F, Puri R, Janssen WG, Rapp PR, Morrison JH. Estrogen restores multisynaptic boutons in the dorsolateral prefrontal cortex while promoting working memory in aged rhesus monkeys. J Neurosci. 2016;36(3):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474. Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Finch CE. Increased synaptic sprouting in response to estrogen via an apolipoprotein E-dependent mechanism: implications for Alzheimer’s disease. J Neurosci. 1998;18(9):3180–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475. Sohrabji F, Lewis DK. Estrogen-BDNF interactions: implications for neurodegenerative diseases. Front Neuroendocrinol. 2006;27(4):404–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 476. Morgan TE, Finch CE. Astrocytic estrogen receptors and impaired neurotrophic responses in a rat model of perimenopause. Front Aging Neurosci. 2015;7:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477. Barreto G, Santos-Galindo M, Diz-Chaves Y, et al. Selective estrogen receptor modulators decrease reactive astrogliosis in the injured brain: effects of aging and prolonged depletion of ovarian hormones. Endocrinology. 2009;150(11):5010–5015. [DOI] [PubMed] [Google Scholar]
- 478. Struble RG, Nathan BP, Cady C, Cheng X, McAsey M. Estradiol regulation of astroglia and apolipoprotein E: an important role in neuronal regeneration. Exp Gerontol. 2007;42(1–2):54–63. [DOI] [PubMed] [Google Scholar]
- 479. Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer’s disease patients. J Neurochem. 2002;80(5):807–814. [DOI] [PubMed] [Google Scholar]
- 480. Vegeto E, Pollio G, Ciana P, Maggi A. Estrogen blocks inducible nitric oxide synthase accumulation in LPS-activated microglia cells. Exp Gerontol. 2000;35(9-10):1309–1316. [DOI] [PubMed] [Google Scholar]
- 481. Vegeto E, Belcredito S, Etteri S, et al. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc Natl Acad Sci U S A. 2003;100(16):9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482. Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147(5):2263–2272. [DOI] [PubMed] [Google Scholar]
- 483. Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A. Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep. 2015;5:15224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484. Cole PA, Robinson CH. Conversion of 19-oxo[2 beta-2H]androgens into oestrogens by human placental aromatase. An unexpected stereochemical outcome. Biochem J. 1990;268(3):553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 485. Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232(4753):1004–1007. [DOI] [PubMed] [Google Scholar]
- 486. Li R, Singh M. Sex differences in cognitive impairment and Alzheimer’s disease. Front Neuroendocrinol. 2014;35(3):385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487. Janicki SC, Schupf N. Hormonal influences on cognition and risk for Alzheimer’s disease. Curr Neurol Neurosci Rep. 2010;10(5):359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488. Janicki SC, Park N, Cheng R, Schupf N, Clark LN, Lee JH. Aromatase variants modify risk for Alzheimer’s disease in a multiethnic female cohort. Dement Geriatr Cogn Disord. 2013;35(5-6):340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489. Ishunina TA, Swaab DF. Decreased alternative splicing of estrogen receptor-α mRNA in the Alzheimer’s disease brain. Neurobiol Aging. 2012;33(2):286–296.e3. [DOI] [PubMed] [Google Scholar]
- 490. Ishunina TA, Swaab DF, Fischer DF. Estrogen receptor-alpha splice variants in the medial mamillary nucleus of Alzheimer’s disease patients: identification of a novel MB1 isoform. J Clin Endocrinol Metab. 2005;90(6):3757–3765. [DOI] [PubMed] [Google Scholar]
- 491. Brandi ML, Becherini L, Gennari L, et al. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer’s disease. Biochem Biophys Res Commun. 1999;265(2):335–338. [DOI] [PubMed] [Google Scholar]
- 492. Mattila KM, Axelman K, Rinne JO, et al. Interaction between estrogen receptor 1 and the epsilon4 allele of apolipoprotein E increases the risk of familial Alzheimer’s disease in women. Neurosci Lett. 2000;282(1-2):45–48. [DOI] [PubMed] [Google Scholar]
- 493. Maruyama H, Toji H, Harrington CR, et al. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57(2):236–240. [DOI] [PubMed] [Google Scholar]
- 494. Pirskanen M, Hiltunen M, Mannermaa A, et al. Estrogen receptor beta gene variants are associated with increased risk of Alzheimer’s disease in women. Eur J Hum Genet. 2005;13(9):1000–1006. [DOI] [PubMed] [Google Scholar]
- 495. Lambert JC, Harris JM, Mann D, et al. Are the estrogen receptors involved in Alzheimer’s disease? Neurosci Lett. 2001;306(3):193–197. [DOI] [PubMed] [Google Scholar]
- 496. Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54(10):1949–1954. [DOI] [PubMed] [Google Scholar]
- 497. Fernández-Martínez M, Elcoroaristizabal Martín X, Blanco Martín E, et al. Oestrogen receptor polymorphisms are an associated risk factor for mild cognitive impairment and Alzheimer disease in women APOE {varepsilon}4 carriers: a case-control study. BMJ Open. 2013;3(9):e003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498. Goodenough S, Schäfer M, Behl C. Estrogen-induced cell signalling in a cellular model of Alzheimer’s disease. J Steroid Biochem Mol Biol. 2003;84(2-3):301–305. [DOI] [PubMed] [Google Scholar]
- 499. Carroll JC, Pike CJ. Selective estrogen receptor modulators differentially regulate Alzheimer-like changes in female 3xTg-AD mice. Endocrinology. 2008;149(5):2607–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500. Carroll JC, Rosario ER, Chang L, et al. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27(48):13357–13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 501. Levin-Allerhand JA, Smith JD. Ovariectomy of young mutant amyloid precursor protein transgenic mice leads to increased mortality. J Mol Neurosci. 2002;19(1-2):163–166. [DOI] [PubMed] [Google Scholar]
- 502. Yue X, Lu M, Lancaster T, et al. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc Natl Acad Sci U S A. 2005;102(52):19198–19203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 503. Petanceska SS, Nagy V, Frail D, Gandy S. Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer’s amyloid beta peptides in brain. Exp Gerontol. 2000;35(9-10):1317–1325. [DOI] [PubMed] [Google Scholar]
- 504. Nord LC, Sundqvist J, Andersson E, Fried G. Analysis of oestrogen regulation of alpha-, beta- and gamma-secretase gene and protein expression in cultured human neuronal and glial cells. Neurodegener Dis. 2010;7(6):349–364. [DOI] [PubMed] [Google Scholar]
- 505. Fernandez JW, Rezai-Zadeh K, Obregon D, Tan J. EGCG functions through estrogen receptor-mediated activation of ADAM10 in the promotion of non-amyloidogenic processing of APP. FEBS Lett. 2010;584(19):4259–4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506. Espinoza I, Caskey M, Baker RC, Miele L. Regulation of Notch localization by endocrine therapy in Estrogen Receptor positive breast cancer cells: Clinical implications for endocrine resistance. Cancer Res. 2012;72(24 Suppl 3). [Google Scholar]
- 507. Crameri A, Biondi E, Kuehnle K, et al. The role of seladin-1/DHCR24 in cholesterol biosynthesis, APP processing and Abeta generation in vivo. Embo J. 2006;25(2):432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508. Migliore L, Fontana I, Trippi F, et al. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging. 2005;26(5):567–573. [DOI] [PubMed] [Google Scholar]
- 509. Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 2010;1359:281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510. Maier M, Peng Y, Jiang L, Seabrook TJ, Carroll MC, Lemere CA. Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008;28(25):6333–6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511. Fan JD, Wagner BL, McDonnell DP. Identification of the sequences within the human complement 3 promoter required for estrogen responsiveness provides insight into the mechanism of tamoxifen mixed agonist activity. Mol Endocrinol. 1996;10(12):1605–1616. [DOI] [PubMed] [Google Scholar]
- 512. Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J Neurochem. 2000;75(4):1447–1454. [DOI] [PubMed] [Google Scholar]
- 513. Reed JL, Dimayuga FO, Davies LM, Keller JN, Bruce-Keller AJ. Estrogen increases proteasome activity in murine microglial cells. Neurosci Lett. 2004;367(1):60–65. [DOI] [PubMed] [Google Scholar]
- 514. Fitzpatrick JL, Mize AL, Wade CB, Harris JA, Shapiro RA, Dorsa DM. Estrogen-mediated neuroprotection against beta-amyloid toxicity requires expression of estrogen receptor alpha or beta and activation of the MAPK pathway. J Neurochem. 2002;82(3):674–682. [DOI] [PubMed] [Google Scholar]
- 515. Harris-White ME, Chu T, Miller SA, Simmons M, et al. Estrogen (E2) and glucocorticoid (Gc) effects on microglia and A beta clearance in vitro and in vivo. Neurochem Int. 2001;39(5-6):435–448. [DOI] [PubMed] [Google Scholar]
- 516. Mangold CA, Wronowski B, Du M, et al. Sexually divergent induction of microglial-associated neuroinflammation with hippocampal aging. J Neuroinflammation. 2017;14(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 517. Vanhanen M, Koivisto K, Moilanen L, et al. Association of metabolic syndrome with Alzheimer disease: a population-based study. Neurology. 2006;67(5):843–847. [DOI] [PubMed] [Google Scholar]
- 518. Della Torre S, Mitro N, Fontana R, et al. An essential role for liver ERα in coupling hepatic metabolism to the reproductive cycle. Cell Rep. 2016;15(2):360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 519. Villa A, Della Torre S, Stell A, Cook J, Brown M, Maggi A. Tetradian oscillation of estrogen receptor α is necessary to prevent liver lipid deposition. Proc Natl Acad Sci U S A. 2012;109(29):11806–11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520. Benedusi V, Martini E, Kallikourdis M, Villa A, Meda C, Maggi A. Ovariectomy shortens the life span of female mice. Oncotarget. 2015;6(13):10801–10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 521. Maric T, Woodside B, Luheshi GN. The effects of dietary saturated fat on basal hypothalamic neuroinflammation in rats. Brain Behav Immun. 2014;36:35–45. [DOI] [PubMed] [Google Scholar]
- 522. Milanski M, Degasperi G, Coope A, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523. Pistell PJ, Morrison CD, Gupta S, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219(1-2):25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524. Erion JR, Wosiski-Kuhn M, Dey A, et al. Obesity elicits interleukin 1-mediated deficits in hippocampal synaptic plasticity. J Neurosci. 2014;34(7):2618–2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525. Cheng X, McAsey ME, Li M, et al. Estradiol replacement increases the low-density lipoprotein receptor related protein (LRP) in the mouse brain. Neurosci Lett. 2007;417(1):50–54. [DOI] [PubMed] [Google Scholar]
- 526. Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129(1):64–69. [DOI] [PubMed] [Google Scholar]
- 527. Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–168. [DOI] [PubMed] [Google Scholar]
- 528. Hoffman GE, Moore N, Fiskum G, Murphy AZ. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp Neurol. 2003;182(1):124–134. [DOI] [PubMed] [Google Scholar]
- 529. De Nicola AF, Gonzalez SL, Labombarda F, et al. Progesterone treatment of spinal cord injury: effects on receptors, neurotrophins, and myelination. J Mol Neurosci. 2006;28(1):3–15. [DOI] [PubMed] [Google Scholar]
- 530. Azcoitia I, Fernandez-Galaz C, Sierra A, Garcia-Segura LM. Gonadal hormones affect neuronal vulnerability to excitotoxin-induced degeneration. J Neurocytol. 1999;28(9):699–710. [DOI] [PubMed] [Google Scholar]
- 531. Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24(10):1160–1166. [DOI] [PubMed] [Google Scholar]
- 532. Rosario ER, Ramsden M, Pike CJ. Progestins inhibit the neuroprotective effects of estrogen in rat hippocampus. Brain Res. 2006;1099(1):206–210. [DOI] [PubMed] [Google Scholar]
- 533. Roof RL, Hoffman SW, Stein DG. Progesterone protects against lipid peroxidation following traumatic brain injury in rats. Mol Chem Neuropathol. 1997;31(1):1–11. [DOI] [PubMed] [Google Scholar]
- 534. Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049(1):112–119. [DOI] [PubMed] [Google Scholar]
- 535. Cervantes M, González-Vidal MD, Ruelas R, Escobar A, Moralí G. Neuroprotective effects of progesterone on damage elicited by acute global cerebral ischemia in neurons of the caudate nucleus. Arch Med Res. 2002;33(1):6–14. [DOI] [PubMed] [Google Scholar]
- 536. Kaur P, Jodhka PK, Underwood WA, et al. Progesterone increases brain-derived neuroptrophic factor expression and protects against glutamate toxicity in a mitogen-activated protein kinase- and phosphoinositide-3 kinase-dependent manner in cerebral cortical explants. J Neurosci Res. 2007;85(11):2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537. Nilsen J, Brinton RD. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002;13(6):825–830. [DOI] [PubMed] [Google Scholar]
- 538. Goodman Y, Bruce AJ, Cheng B, Mattson MP. Estrogens attenuate and corticosterone exacerbates excitotoxicity, oxidative injury, and amyloid beta-peptide toxicity in hippocampal neurons. J Neurochem. 1996;66(5):1836–1844. [DOI] [PubMed] [Google Scholar]
- 539. Su C, Cunningham RL, Rybalchenko N, Singh M. Progesterone increases the release of brain-derived neurotrophic factor from glia via progesterone receptor membrane component 1 (Pgrmc1)-dependent ERK5 signaling. Endocrinology. 2012;153(9):4389–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 540. Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000;101(4):931–938. [DOI] [PubMed] [Google Scholar]
- 541. Zandi PP, Carlson MC, Plassman BL, et al. ; Cache County Memory Study Investigators . Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. Jama. 2002;288(17):2123–2129. [DOI] [PubMed] [Google Scholar]
- 542. Waring SC, Rocca WA, Petersen RC, O’Brien PC, Tangalos EG, Kokmen E. Postmenopausal estrogen replacement therapy and risk of AD: a population-based study. Neurology. 1999;52(5):965–970. [DOI] [PubMed] [Google Scholar]
- 543. Tang MX, Jacobs D, Stern Y, et al. Effect of oestrogen during menopause on risk and age at onset of Alzheimer’s disease. Lancet. 1996;348(9025):429–432. [DOI] [PubMed] [Google Scholar]
- 544. Almeida S, Fiegenbaum M, de Andrade FM, Osório-Wender MC, Hutz MH. ESR1 and APOE gene polymorphisms, serum lipids, and hormonal replacement therapy. Maturitas. 2006;54(2):119–126. [DOI] [PubMed] [Google Scholar]
- 545. Yaffe K. Estrogens, selective estrogen receptor modulators, and dementia: what is the evidence? Ann N Y Acad Sci. 2001;949:215–222. [DOI] [PubMed] [Google Scholar]
- 546. Mulnard RA, Cotman CW, Kawas C, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s disease Cooperative Study. Jama. 2000;283(8):1007–1015. [DOI] [PubMed] [Google Scholar]
- 547. Shao H, Breitner JC, Whitmer RA, et al. ; Cache County Investigators . Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology. 2012;79(18):1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548. Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm AC. Progesterone reverses the spatial memory enhancements initiated by tonic and cyclic oestrogen therapy in middle-aged ovariectomized female rats. Eur J Neurosci. 2006;24(1):229–242. [DOI] [PubMed] [Google Scholar]
- 549. O’Brien J, Jackson JW, Grodstein F, Blacker D, Weuve J. Postmenopausal hormone therapy is not associated with risk of all-cause dementia and Alzheimer’s disease. Epidemiol Rev. 2014;36:83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550. Morley JE, Kaiser FE, Perry HM 3rd, et al. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46(4):410–413. [DOI] [PubMed] [Google Scholar]
- 551. Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–876. [DOI] [PubMed] [Google Scholar]
- 552. Rosario ER, Chang L, Beckett TL, et al. Age-related changes in serum and brain levels of androgens in male Brown Norway rats. Neuroreport. 2009;20(17):1534–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553. Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011;32(4):604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554. Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone depletion and the development of Alzheimer disease. Jama. 2004;292(12):1431–1432. [DOI] [PubMed] [Google Scholar]
- 555. Hogervorst E, Combrinck M, Smith AD. Testosterone and gonadotropin levels in men with dementia. Neuro Endocrinol Lett. 2003;24(3-4):203–208. [PubMed] [Google Scholar]
- 556. Moffat SD, Zonderman AB, Metter EJ, et al. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62(2):188–193. [DOI] [PubMed] [Google Scholar]
- 557. Wahjoepramono EJ, Wijaya LK, Taddei K, et al. Distinct effects of testosterone on plasma and cerebrospinal fluid amyloid-beta levels. J Alzheimers Dis. 2008;15(1):129–137. [DOI] [PubMed] [Google Scholar]
- 558. Brännvall K, Bogdanovic N, Korhonen L, Lindholm D. 19-Nortestosterone influences neural stem cell proliferation and neurogenesis in the rat brain. Eur J Neurosci. 2005;21(4):871–878. [DOI] [PubMed] [Google Scholar]
- 559. Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64(1):34–46. [DOI] [PubMed] [Google Scholar]
- 560. Matsumoto A. Androgen stimulates neuronal plasticity in the perineal motoneurons of aged male rats. J Comp Neurol. 2001;430(3):389–395. [DOI] [PubMed] [Google Scholar]
- 561. García-Segura LM, Chowen JA, Párducz A, Naftolin F. Gonadal hormones as promoters of structural synaptic plasticity: cellular mechanisms. Prog Neurobiol. 1994;44(3):279–307. [DOI] [PubMed] [Google Scholar]
- 562. Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav. 2008;53(5):638–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563. Huppenbauer CB, Tanzer L, DonCarlos LL, Jones KJ. Gonadal steroid attenuation of developing hamster facial motoneuron loss by axotomy: equal efficacy of testosterone, dihydrotestosterone, and 17-beta estradiol. J Neurosci. 2005;25(16):4004–4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 564. Yu WH. Sex difference in neuronal loss induced by axotomy in the rat brain stem motor nuclei. Exp Neurol. 1988;102(2):230–235. [DOI] [PubMed] [Google Scholar]
- 565. Brooks BP, Merry DE, Paulson HL, Lieberman AP, Kolson DL, Fischbeck KH. A cell culture model for androgen effects in motor neurons. J Neurochem. 1998;70(3):1054–1060. [DOI] [PubMed] [Google Scholar]
- 566. Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77(5):1319–1326. [DOI] [PubMed] [Google Scholar]
- 567. Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892(2):255–262. [DOI] [PubMed] [Google Scholar]
- 568. Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23(5):1588–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569. MacLusky NJ, Hajszan T, Leranth C. Effects of dehydroepiandrosterone and flutamide on hippocampal CA1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology. 2004;145(9):4154–4161. [DOI] [PubMed] [Google Scholar]
- 570. Nguyen TV, Yao M, Pike CJ. Androgens activate mitogen-activated protein kinase signaling: role in neuroprotection. J Neurochem. 2005;94(6):1639–1651. [DOI] [PubMed] [Google Scholar]
- 571. Park SY, Tournell C, Sinjoanu RC, Ferreira A. Caspase-3- and calpain-mediated tau cleavage are differentially prevented by estrogen and testosterone in beta-amyloid-treated hippocampal neurons. Neuroscience. 2007;144(1):119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 572. Lei Y, Renyuan Z. Effects of androgens on the amyloid-β protein in Alzheimer’s disease. Endocrinology. 2018;159(12):3885–3894. [DOI] [PubMed] [Google Scholar]
- 573. Buskbjerg CR, Gravholt CH, Dalby HR, Amidi A, Zachariae R. Testosterone supplementation and cognitive functioning in men-a systematic review and meta-analysis. J Endocr Soc. 2019;3(8):1465–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 574. Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62(2):155–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 575. Küppers E, Ivanova T, Karolczak M, Beyer C. Estrogen: a multifunctional messenger to nigrostriatal dopaminergic neurons. J Neurocytol. 2000;29(5-6):375–385. [DOI] [PubMed] [Google Scholar]
- 576. Beyer C, Pilgrim C, Reisert I. Dopamine content and metabolism in mesencephalic and diencephalic cell cultures: sex differences and effects of sex steroids. J Neurosci. 1991;11(5):1325–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 577. Sánchez MG, Bourque M, Morissette M, Di Paolo T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16(3):e43–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 578. Shughrue PJ. Estrogen attenuates the MPTP-induced loss of dopamine neurons from the mouse SNc despite a lack of estrogen receptors (ERalpha and ERbeta). Exp Neurol. 2004;190(2):468–477. [DOI] [PubMed] [Google Scholar]
- 579. Leranth C, Roth RH, Elsworth JD, Naftolin F, Horvath TL, Redmond DE Jr. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J Neurosci. 2000;20(23):8604–8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580. Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22(4):226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 581. Klink R, Robichaud M, Debonnel G. Gender and gonadal status modulation of dorsal raphe nucleus serotonergic neurons. Part II. Regulatory mechanisms. Neuropharmacology. 2002;43(7):1129–1138. [DOI] [PubMed] [Google Scholar]
- 582. Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59(2):122–124. [DOI] [PubMed] [Google Scholar]
- 583. Almey A, Filardo EJ, Milner TA, Brake WG. Estrogen receptors are found in glia and at extranuclear neuronal sites in the dorsal striatum of female rats: evidence for cholinergic but not dopaminergic colocalization. Endocrinology. 2012;153(11):5373–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584. Schultz KN, von Esenwein SA, Hu M, et al. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29(6):1897–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 585. Quinn NP, Marsden CD. Menstrual-related fluctuations in Parkinson’s disease. Mov Disord. 1986;1(1):85–87. [DOI] [PubMed] [Google Scholar]
- 586. Ragonese P, D’Amelio M, Savettieri G. Implications for estrogens in Parkinson’s disease: an epidemiological approach. Ann N Y Acad Sci. 2006;1089:373–382. [DOI] [PubMed] [Google Scholar]
- 587. Benedetti MD, Maraganore DM, Bower JH, et al. Hysterectomy, menopause, and estrogen use preceding Parkinson’s disease: an exploratory case-control study. Mov Disord. 2001;16(5):830–837. [DOI] [PubMed] [Google Scholar]
- 588. Rocca WA, Bower JH, Maraganore DM, et al. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70(3):200–209. [DOI] [PubMed] [Google Scholar]
- 589. Dye RV, Miller KJ, Singer EJ, Levine AJ. Hormone replacement therapy and risk for neurodegenerative diseases. Int J Alzheimers Dis. 2012;2012:258454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 590. Labandeira-Garcia JL, Rodriguez-Perez AI, Valenzuela R, Costa-Besada MA, Guerra MJ. Menopause and Parkinson’s disease. Interaction between estrogens and brain renin-angiotensin system in dopaminergic degeneration. Front Neuroendocrinol. 2016;43:44–59. [DOI] [PubMed] [Google Scholar]
- 591. Morissette M, Le Saux M, D’Astous M, et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108(3–5):327–338. [DOI] [PubMed] [Google Scholar]
- 592. Moroz IA, Rajabi H, Rodaros D, Stewart J. Effects of sex and hormonal status on astrocytic basic fibroblast growth factor-2 and tyrosine hydroxylase immunoreactivity after medial forebrain bundle 6-hydroxydopamine lesions of the midbrain dopamine neurons. Neuroscience. 2003;118(2):463–476. [DOI] [PubMed] [Google Scholar]
- 593. Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev Neurobiol. 2008;68(5):632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594. Rodriguez-Perez AI, Borrajo A, Valenzuela R, Lanciego JL, Labandeira-Garcia JL. Critical period for dopaminergic neuroprotection by hormonal replacement in menopausal rats. Neurobiol Aging. 2015;36(2):1194–1208. [DOI] [PubMed] [Google Scholar]
- 595. Siani F, Greco R, Levandis G, Ghezzi C, et al. Influence of estrogen modulation on glia activation in a murine model of Parkinson’s disease. Front Neurosci. 2017;11:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596. Côté M, Bourque M, Poirier AA, et al. GPER1-mediated immunomodulation and neuroprotection in the myenteric plexus of a mouse model of Parkinson’s disease. Neurobiol Dis. 2015;82:99–113. [DOI] [PubMed] [Google Scholar]
- 597. Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29(5):241–249. [DOI] [PubMed] [Google Scholar]
- 598. Grassi D, Bellini MJ, Acaz-Fonseca E, Panzica G, Garcia-Segura LM. Estradiol and testosterone regulate arginine-vasopressin expression in SH-SY5Y human female neuroblastoma cells through estrogen receptors-α and -β. Endocrinology. 2013;154(6):2092–2100. [DOI] [PubMed] [Google Scholar]
- 599. Litim N, Morissette M, Di Paolo T. Effects of progesterone administered after MPTP on dopaminergic neurons of male mice. Neuropharmacology. 2017;117:209–218. [DOI] [PubMed] [Google Scholar]
- 600. Yu L, Kuo Y, Cherng CG, Chen HH, Hsu CH. Ovarian hormones do not attenuate methamphetamine-induced dopaminergic neurotoxicity in mice gonadectomized at 4 weeks postpartum. Neuroendocrinology. 2002;75(5):282–287. [DOI] [PubMed] [Google Scholar]
- 601. Woolley SC, O’Malley B, Lydon J, Crews D. Genotype differences in behavior and tyrosine hydroxylase expression between wild-type and progesterone receptor knockout mice. Behav Brain Res. 2006;167(2):197–204. [DOI] [PubMed] [Google Scholar]
- 602. Petitclerc M, Bédard PJ, Di Paolo T. Progesterone releases dopamine in male and female rat striatum: a behavioral and microdialysis study. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(3):491–497. [DOI] [PubMed] [Google Scholar]
- 603. Dluzen DE, Ramirez VD. Effects of orchidectomy on nigro-striatal dopaminergic function: behavioral and physiological evidence. J Neuroendocrinol. 1989;1(4):285–290. [DOI] [PubMed] [Google Scholar]
- 604. Johnson ML, Day AE, Ho CC, Walker QD, Francis R, Kuhn CM. Androgen decreases dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22(4):238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605. Khasnavis S, Ghosh A, Roy A, Pahan K. Castration induces Parkinson disease pathologies in young male mice via inducible nitric-oxide synthase. J Biol Chem. 2013;288(29):20843–20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 606. Kenangil G, Orken DN, Ur E, Forta H, Celik M. The relation of testosterone levels with fatigue and apathy in Parkinson’s disease. Clin Neurol Neurosurg. 2009;111(5):412–414. [DOI] [PubMed] [Google Scholar]
- 607. Mitchell E, Thomas D, Burnet R. Testosterone improves motor function in Parkinson’s disease. J Clin Neurosci. 2006;13(1):133–136. [DOI] [PubMed] [Google Scholar]
- 608. Okun MS, Walter BL, McDonald WM, et al. Beneficial effects of testosterone replacement for the nonmotor symptoms of Parkinson disease. Arch Neurol. 2002;59(11):1750–1753. [DOI] [PubMed] [Google Scholar]
- 609. Okun MS, McDonald WM, DeLong MR. Refractory nonmotor symptoms in male patients with Parkinson disease due to testosterone deficiency: a common unrecognized comorbidity. Arch Neurol. 2002;59(5):807–811. [DOI] [PubMed] [Google Scholar]
- 610. Ekue A, Boulanger JF, Morissette M, Di Paolo T. Lack of effect of testosterone and dihydrotestosterone compared to 17beta-oestradiol in 1-methyl-4-phenyl-1,2,3,6, tetrahydropyridine-mice. J Neuroendocrinol. 2002;14(9):731–736. [DOI] [PubMed] [Google Scholar]
- 611. Litim N, Bourque M, Al Sweidi S, Morissette M, Di Paolo T. The 5α-reductase inhibitor Dutasteride but not Finasteride protects dopamine neurons in the MPTP mouse model of Parkinson’s disease. Neuropharmacology. 2015;97:86–94. [DOI] [PubMed] [Google Scholar]
- 612. Holmes S, Abbassi B, Su C, Singh M, Cunningham RL. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology. 2013;154(11):4281–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 613. Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16(1):17–29. [DOI] [PubMed] [Google Scholar]
- 614. Morale MC, Serra PA, L’episcopo F, et al. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience. 2006;138(3):869–878. [DOI] [PubMed] [Google Scholar]
- 615. Kawano N, Koji T, Hishikawa Y, Murase K, Murata I, Kohno S. Identification and localization of estrogen receptor alpha- and beta-positive cells in adult male and female mouse intestine at various estrogen levels. Histochem Cell Biol. 2004;121(5):399–405. [DOI] [PubMed] [Google Scholar]
- 616. Sipilä S, Finni T, Kovanen V. Estrogen influences on neuromuscular function in postmenopausal women. Calcif Tissue Int. 2015;96(3):222–233. [DOI] [PubMed] [Google Scholar]
- 617. Carson JA, Manolagas SC. Effects of sex steroids on bones and muscles: Similarities, parallels, and putative interactions in health and disease. Bone. 2015;80:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 618. Hansen M. Female hormones: do they influence muscle and tendon protein metabolism? Proc Nutr Soc. 2018;77(1):32–41. [DOI] [PubMed] [Google Scholar]
- 619. Ekenros L, Papoutsi Z, Fridén C, Dahlman Wright K, Lindén Hirschberg A. Expression of sex steroid hormone receptors in human skeletal muscle during the menstrual cycle. Acta Physiol (Oxf). 2017;219(2):486–493. [DOI] [PubMed] [Google Scholar]
- 620. Ribas V, Drew BG, Zhou Z, et al. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med. 2016;8(334):334ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 621. Vivekananda U, Manjalay ZR, Ganesalingam J, et al. Low index-to-ring finger length ratio in sporadic ALS supports prenatally defined motor neuronal vulnerability. J Neurol Neurosurg Psychiatry. 2011;82(6):635–637. [DOI] [PubMed] [Google Scholar]
- 622. Wicks P. Hypothesis: higher prenatal testosterone predisposes ALS patients to improved athletic performance and manual professions. Amyotroph Lateral Scler. 2012;13(3):251–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623. Weiner LP. Possible role of androgen receptors in amyotrophic lateral sclerosis. A hypothesis. Arch Neurol. 1980;37(3):129–131. [DOI] [PubMed] [Google Scholar]
- 624. Simeoni S, Mancini MA, Stenoien DL, et al. Motoneuronal cell death is not correlated with aggregate formation of androgen receptors containing an elongated polyglutamine tract. Hum Mol Genet. 2000;9(1):133–144. [DOI] [PubMed] [Google Scholar]
- 625. Garofalo O, Figlewicz DA, Leigh PN, et al. Androgen receptor gene polymorphisms in amyotrophic lateral sclerosis. Neuromuscul Disord. 1993;3(3):195–199. [DOI] [PubMed] [Google Scholar]
- 626. Horner RD, Kamins KG, Feussner JR, et al. Occurrence of amyotrophic lateral sclerosis among Gulf War veterans. Neurology. 2003;61(6):742–749. [DOI] [PubMed] [Google Scholar]
- 627. Weisskopf MG, O’Reilly EJet al. Prospective study of military service and mortality from ALS. Neurology. 2005;64(1):32–37. [DOI] [PubMed] [Google Scholar]
- 628. Chiò A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005;128(Pt 3):472–476. [DOI] [PubMed] [Google Scholar]
- 629. Belli S, Vanacore N. Proportionate mortality of Italian soccer players: is amyotrophic lateral sclerosis an occupational disease? Eur J Epidemiol. 2005;20(3):237–242. [DOI] [PubMed] [Google Scholar]
- 630. Beghi E. Are professional soccer players at higher risk for ALS? Amyotroph Lat Scl Fr. 2013;14(7–8):501–506. [DOI] [PubMed] [Google Scholar]
- 631. Giorgetti E, Rusmini P, Crippa Vet al. Synergic prodegradative activity of Bicalutamide and trehalose on the mutant androgen receptor responsible for spinal and bulbar muscular atrophy. Hum Mol Genet. 2015;24(1):64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632. Rusmini P, Crippa V, Cristofani R, et al. The role of the protein quality control system in SBMA. J Mol Neurosci. 2016;58(3):348–364. [DOI] [PubMed] [Google Scholar]
- 633. Gargiulo-Monachelli GM, Sivori M, Meyer M, et al. Circulating gonadal and adrenal steroids in amyotrophic lateral sclerosis: possible markers of susceptibility and outcome. Horm Metab Res. 2014;46(6):433–439. [DOI] [PubMed] [Google Scholar]
- 634. Galbiati M, Onesto E, Zito A, et al. The anabolic/androgenic steroid nandrolone exacerbates gene expression modifications induced by mutant SOD1 in muscles of mice models of amyotrophic lateral sclerosis. Pharmacol Res. 2012;65(2):221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635. Galbiati M, Crippa V, Rusmini Pet al. ALS-related misfolded protein management in motor neurons and muscle cells. Neurochem Int. 2014;79:70–78. [DOI] [PubMed] [Google Scholar]
- 636. Aggarwal T, Polanco MJ, Scaramuzzino C, et al. Androgens affect muscle, motor neuron, and survival in a mouse model of SOD1-related amyotrophic lateral sclerosis. Neurobiol Aging. 2014;35(8):1929–1938. [DOI] [PubMed] [Google Scholar]
- 637. McLeod VM, Lau CL, Chiam MDF, et al. Androgen receptor antagonism accelerates disease onset in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Br J Pharmacol. 2019;176(13):2111–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638. Crippa V, Boncoraglio A, Galbiati M, et al. Differential autophagy power in the spinal cord and muscle of transgenic ALS mice. Front Cell Neurosci. 2013;7:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 639. Crippa V, Galbiati M, Boncoraglio A, et al. Motoneuronal and muscle-selective removal of ALS-related misfolded proteins. Biochem Soc Trans. 2013;41(6):1598–1604. [DOI] [PubMed] [Google Scholar]
- 640. Kim J, Kim TY, Cho KS, Kim HN, Koh JY. Autophagy activation and neuroprotection by progesterone in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2013;59:80–85. [DOI] [PubMed] [Google Scholar]
- 641. Schmitt-John T, Drepper C, Mussmann A, et al. Mutation of Vps54 causes motor neuron disease and defective spermiogenesis in the wobbler mouse. Nat Genet. 2005;37(11):1213–1215. [DOI] [PubMed] [Google Scholar]
- 642. González Deniselle MC, Garay L, López-Costa JJ, et al. Progesterone treatment reduces NADPH-diaphorase/nitric oxide synthase in Wobbler mouse motoneuron disease. Brain Res. 2004;1014(1-2):71–79. [DOI] [PubMed] [Google Scholar]
- 643. Gonzalez Deniselle MC, Garay L, Gonzalez S, Guennoun R, Schumacher M, De Nicola AF. Progesterone restores retrograde labeling of cervical motoneurons in Wobbler mouse motoneuron disease. Exp Neurol. 2005;195(2):518–523. [DOI] [PubMed] [Google Scholar]
- 644. Gonzalez Deniselle MC, Garay L, Gonzalez S, et al. Progesterone modulates brain-derived neurotrophic factor and choline acetyltransferase in degenerating Wobbler motoneurons. Exp Neurol. 2007;203(2):406–414. [DOI] [PubMed] [Google Scholar]
- 645. Gonzalez Deniselle MC, Liere P, Pianos A, et al. Steroid profiling in male Wobbler mouse, a model of amyotrophic lateral sclerosis. Endocrinology. 2016;157(11):4446–4460. [DOI] [PubMed] [Google Scholar]
- 646. Meyer M, Gonzalez Deniselle MC, Garay L, et al. The progesterone receptor agonist Nestorone holds back proinflammatory mediators and neuropathology in the wobbler mouse model of motoneuron degeneration. Neuroscience. 2015;308:51–63. [DOI] [PubMed] [Google Scholar]
- 647. De Nicola AF, Coronel F, Garay LI, et al. Therapeutic effects of progesterone in animal models of neurological disorders. CNS Neurol Disord Drug Targets. 2013;12(8):1205–1218. [PubMed] [Google Scholar]
- 648. Gargiulo-Monachelli GM, Campos-Melo D, Droppelmann CA, et al. Expression and cellular localization of the classical progesterone receptor in healthy and amyotrophic lateral sclerosis affected spinal cord. Eur J Neurol. 2014;21(2):273–80.e11. [DOI] [PubMed] [Google Scholar]
- 649. Bame M, Pentiak PA, Needleman R, Brusilow WS. Effect of sex on lifespan, disease progression, and the response to methionine sulfoximine in the SOD1 G93A mouse model for ALS. Gend Med. 2012;9(6):524–535. [DOI] [PubMed] [Google Scholar]
- 650. Cardona-Rossinyol A, Mir M, Caraballo-Miralles V, Lladó J, Olmos G. Neuroprotective effects of estradiol on motoneurons in a model of rat spinal cord embryonic explants. Cell Mol Neurobiol. 2013;33(3):421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651. Sun C, Liu Y, Liu Y, et al. Characterization of aromatase expression in the spinal cord of an animal model of familial ALS. Brain Res Bull. 2017;132:180–189. [DOI] [PubMed] [Google Scholar]
- 652. Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Estrogen and cytokines production - the possible cause of gender differences in neurological diseases. Curr Pharm Des. 2005;11(8):1017–1030. [DOI] [PubMed] [Google Scholar]
- 653. Mhatre M, Floyd RA, Hensley K. Oxidative stress and neuroinflammation in Alzheimer’s disease and amyotrophic lateral sclerosis: common links and potential therapeutic targets. J Alzheimers Dis. 2004;6(2):147–157. [DOI] [PubMed] [Google Scholar]
- 654. Hensley K, Fedynyshyn J, Ferrell S, et al. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14(1):74–80. [DOI] [PubMed] [Google Scholar]
- 655. Babu GN, Kumar A, Chandra R, Puri SK, Kalita J, Misra UK. Elevated inflammatory markers in a group of amyotrophic lateral sclerosis patients from northern India. Neurochem Res. 2008;33(6):1145–1149. [DOI] [PubMed] [Google Scholar]
- 656. Das A, Smith JA, Gibson C, Varma AK, Ray SK, Banik NL. Estrogen receptor agonists and estrogen attenuate TNF-α-induced apoptosis in VSC4.1 motoneurons. J Endocrinol. 2011;208(2):171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 657. Zhao W, Varghese M, Yemul S, et al. Peroxisome proliferator activator receptor gamma coactivator-1alpha (PGC-1α) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2011;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 658. Thau N, Knippenberg S, Körner S, Rath KJ, Dengler R, Petri S. Decreased mRNA expression of PGC-1α and PGC-1α-regulated factors in the SOD1G93A ALS mouse model and in human sporadic ALS. J Neuropathol Exp Neurol. 2012;71(12):1064–1074. [DOI] [PubMed] [Google Scholar]
- 659. Eschbach J, Schwalenstöcker B, Soyal SM, et al. PGC-1α is a male-specific disease modifier of human and experimental amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22(17):3477–3484. [DOI] [PubMed] [Google Scholar]
- 660. Vieira E, Marroquí L, Figueroa AL, et al. Involvement of the clock gene Rev-erb alpha in the regulation of glucagon secretion in pancreatic alpha-cells. PloS One. 2013;8(7):e69939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661. Kaneb HM, Sharp PS, Rahmani-Kondori N, Wells DJ. Metformin treatment has no beneficial effect in a dose-response survival study in the SOD1(G93A) mouse model of ALS and is harmful in female mice. PloS One. 2011;6(9):e24189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 662. Kemper MF, Zhao Y, Duckles SP, Krause DN. Endogenous ovarian hormones affect mitochondrial efficiency in cerebral endothelium via distinct regulation of PGC-1 isoforms. J Cereb Blood Flow Metab. 2013;33(1):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 663. Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139(9):4008–4011. [DOI] [PubMed] [Google Scholar]
- 664. Yang JW, Kim SM, Kim HJ, et al. Hypolipidemia in patients with amyotrophic lateral sclerosis: a possible gender difference? J Clin Neurol. 2013;9(2):125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 665. Nefussy B, Hirsch J, Cudkowicz ME, Drory VE. Gender-based effect of statins on functional decline in amyotrophic lateral sclerosis. J Neurol Sci. 2011;300(1-2):23–27. [DOI] [PubMed] [Google Scholar]
- 666. Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol. 2000;20(5):1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 667. Benedusi V, Meda C, Della Torre S, Monteleone G, Vegeto E, Maggi A. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology. 2012;153(6):2777–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 668. Chu R, van Hasselt A, Vlantis AC, et al. The cross-talk between estrogen receptor and peroxisome proliferator-activated receptor gamma in thyroid cancer. Cancer. 2014;120(1):142–153. [DOI] [PubMed] [Google Scholar]
- 669. Cacabelos D, Ayala V, Ramírez-Nunez O, et al. Dietary lipid unsaturation influences survival and oxidative modifications of an amyotrophic lateral sclerosis model in a gender-specific manner. Neuromolecular Med. 2014;16(4):669–685. [DOI] [PubMed] [Google Scholar]
- 670. Cacabelos D, Ramírez-Núñez O, Granado-Serrano AB, et al. Early and gender-specific differences in spinal cord mitochondrial function and oxidative stress markers in a mouse model of ALS. Acta Neuropathol Commun. 2016;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 671. Riar AK, Burstein SR, Palomo GM, Arreguin A, Manfredi G, Germain D. Sex specific activation of the ERα axis of the mitochondrial UPR (UPRmt) in the G93A-SOD1 mouse model of familial ALS. Hum Mol Genet. 2017;26(7):1318–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 672. Piccolella M, Crippa V, Cristofani R, et al. The small heat shock protein B8 (HSPB8) modulates proliferation and migration of breast cancer cells. Oncotarget. 2017;8(6):10400–10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 673. Rusmini P, Cristofani R, Galbiati M, et al. The role of the heat shock protein B8 (HSPB8) in motoneuron diseases. Front Mol Neurosci. 2017;10:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 674. Cristofani R, Crippa V, Vezzoli G, et al. The small heat shock protein B8 (HSPB8) efficiently removes aggregating species of dipeptides produced in C9ORF72-related neurodegenerative diseases. Cell Stress Chaperones. 2018;23(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 675. Deng L, Feng J, Broaddus RR. The novel estrogen-induced gene EIG121 regulates autophagy and promotes cell survival under stress. Cell Death Dis. 2010;1:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 676. Crippa V, Carra S, Rusmini P, et al. A role of small heat shock protein B8 (HspB8) in the autophagic removal of misfolded proteins responsible for neurodegenerative diseases. Autophagy. 2010;6(7):958–960. [DOI] [PubMed] [Google Scholar]
- 677. Crippa V, Cicardi ME, Ramesh N, et al. The chaperone HSPB8 reduces the accumulation of truncated TDP-43 species in cells and protects against TDP-43-mediated toxicity. Hum Mol Genet. 2016;25(18):3908–3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 678. Cristofani R, Crippa V, Rusmini P, et al. Inhibition of retrograde transport modulates misfolded protein accumulation and clearance in motoneuron diseases. Autophagy. 2017;13(8):1280–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679. Sun X, Fontaine JM, Bartl I, Behnam B, Welsh MJ, Benndorf R. Induction of Hsp22 (HspB8) by estrogen and the metalloestrogen cadmium in estrogen receptor-positive breast cancer cells. Cell Stress Chaperones. 2007;12(4):307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680. Nance MA. Clinical aspects of CAG repeat diseases. Brain Pathol. 1997;7(3):881–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681. Fernández-Rhodes LE, Kokkinis AD, White MJ, et al. Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomised placebo-controlled trial. Lancet Neurol. 2011;10(2):140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 682. Katsuno M, Tanaka F, Adachi H, et al. Pathogenesis and therapy of spinal and bulbar muscular atrophy (SBMA). Prog Neurobiol. 2012;99(3):246–256. [DOI] [PubMed] [Google Scholar]
- 683. Fischbeck KH. Developing treatment for spinal and bulbar muscular atrophy. Prog Neurobiol. 2012;99(3):257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684. Lanman TA, Bakar D, Badders NM, et al. Sexual reassignment fails to prevent Kennedy’s disease. J Neuromuscul Dis. 2016;3(1):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 685. Montie HL, Pestell RG, Merry DE. SIRT1 modulates aggregation and toxicity through deacetylation of the androgen receptor in cell models of SBMA. J Neurosci. 2011;31(48):17425–17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 686. Pennuto M, Palazzolo I, Poletti A. Post-translational modifications of expanded polyglutamine proteins: impact on neurotoxicity. Hum Mol Genet. 2009;18(R1):R40–R47. [DOI] [PubMed] [Google Scholar]
- 687. Rusmini P, Sau D, Crippa V, et al. Aggregation and proteasome: the case of elongated polyglutamine aggregation in spinal and bulbar muscular atrophy. Neurobiol Aging. 2007;28(7):1099–1111. [DOI] [PubMed] [Google Scholar]
- 688. Rusmini P, Crippa V, Giorgetti E, et al. Clearance of the mutant androgen receptor in motoneuronal models of spinal and bulbar muscular atrophy. Neurobiol Aging. 2013;34(11):2585–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 689. Darrington RS, Butler R, Leigh PN, McPhaul MJ, Gallo JM. Ligand-dependent aggregation of polyglutamine-expanded androgen receptor in neuronal cells. Neuroreport. 2002;13(16):2117–2120. [DOI] [PubMed] [Google Scholar]
- 690. Renier KJ, Troxell-Smith SM, Johansen JA, et al. Antiandrogen flutamide protects male mice from androgen-dependent toxicity in three models of spinal bulbar muscular atrophy. Endocrinology. 2014;155(7):2624–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691. Badders NM, Korff A, Miranda HC, et al. Selective modulation of the androgen receptor AF2 domain rescues degeneration in spinal bulbar muscular atrophy. Nat Med. 2018;24(4):427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 692. Adachi H, Waza M, Tokui K, et al. CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. J Neurosci. 2007;27(19):5115–5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 693. Montie HL, Cho MS, Holder L, et al. Cytoplasmic retention of polyglutamine-expanded androgen receptor ameliorates disease via autophagy in a mouse model of spinal and bulbar muscular atrophy. Hum Mol Genet. 2009;18(11):1937–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 694. Rusmini P, Simonini F, Crippa V, et al. 17-AAG increases autophagic removal of mutant androgen receptor in spinal and bulbar muscular atrophy. Neurobiol Dis. 2011;41(1):83–95. [DOI] [PubMed] [Google Scholar]