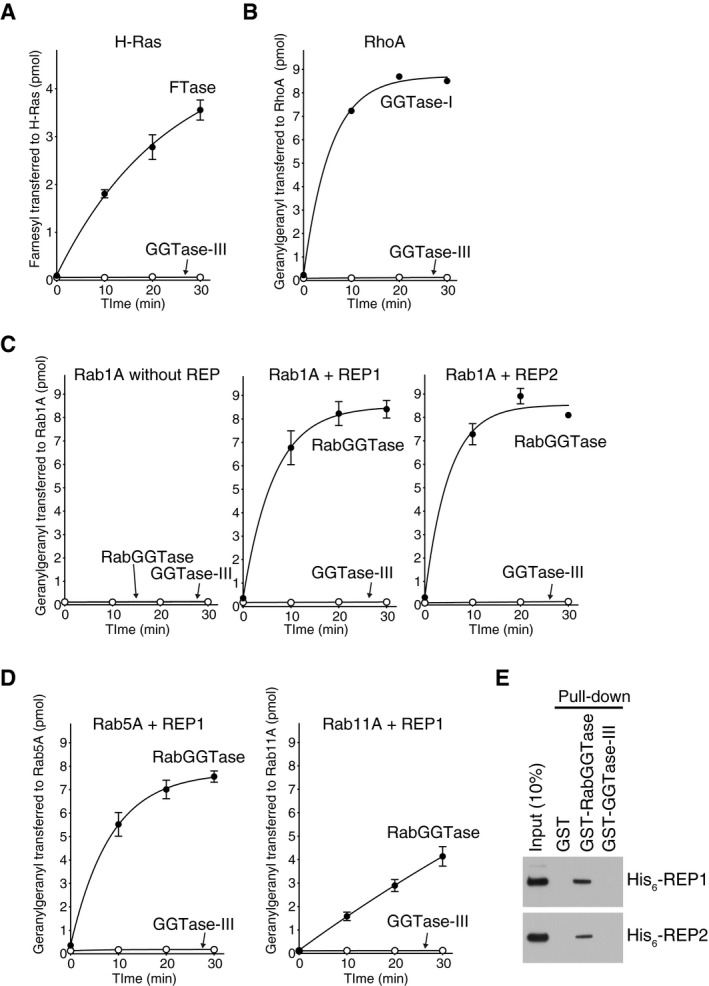
Figure EV1. GGTase‐III has no prenyltransferase activity on known prenyltransferase substrates.
-
A–DPrenylation activity of GGTase‐III on Ras, Rho, and Rab proteins. (A) His6‐H‐Ras (5 μM) was incubated with FTase (50 nM) or GGTase‐III (50 nM) and 3H‐FPP (1 μM) at 37°C. Reactions were stopped at the indicated time points, and the amount of 3H‐farnesyl transferred to H‐Ras was quantified by scintillation counting (mean ± SEM, n = 3). (B) His6‐RhoA (5 μM) was incubated with GGTase‐I (50 nM) or GGTase‐III (50 nM) and 3H‐GGPP (1 μM), and the amount of 3H‐geranylgeranyl transferred to RhoA was quantified (mean ± SEM, n = 3). (C) His6‐Rab1A (5 μM) was incubated with RabGGTase (50 nM) or GGTase‐III (50 nM) and 3H‐GGPP (1 μM) in the absence or presence of either His6‐REP1 (100 nM) or His6‐REP2 (100 nM), and the amount of 3H‐geranylgeranyl transferred to Rab1A was quantified (mean ± SEM, n = 3). (D) His6‐Rab5A and His6‐Rab11A were incubated with RabGGTase (50 nM) or GGTase‐III (50 nM) and 3H‐GGPP (1 μM) in the presence of His6‐REP1 (100 nM), and the amount of 3H‐geranylgeranyl transferred to Rab5A and Rab11A was quantified (mean ± SEM, n = 3).
-
EREP pull‐down assay. Recombinant His6‐REP1 or His6‐REP2 was incubated with glutathione Sepharose beads coated with GST, GST‐RabGGTase, or GST‐GGTase‐III. Bound His6‐REP proteins were analyzed by immunoblotting with anti‐His6 antibody.
Source data are available online for this figure.
