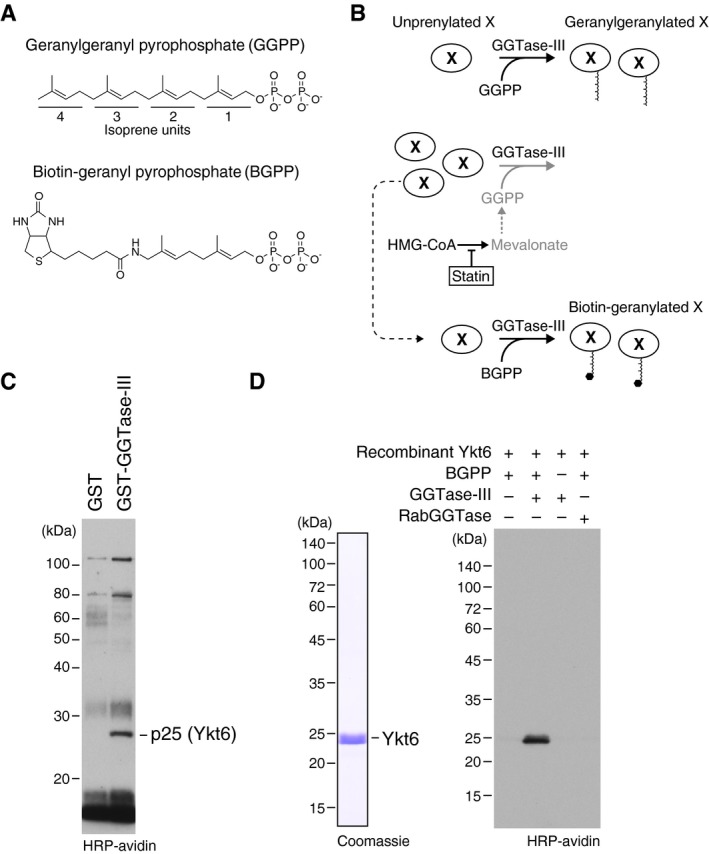
Figure 2. Identification of Ykt6 as a substrate of GGTase‐III .
-
AMolecular structures of geranylgeranyl pyrophosphate (GGPP) and its biotinylated analogue biotin‐geranyl pyrophosphate (BGPP). Geranylgeranyl moiety consists of four repeats of 5‐carbon isoprene unit.
-
BPurification strategy for GGTase‐III substrate proteins using statin and BGPP. “X” represents a putative substrate protein of GGTase‐III. HMG‐CoA, hydroxymethylglutaryl‐CoA.
-
CIdentification of Ykt6 as a protein biotin‐geranylated by GGTase‐III. Cytosolic proteins extracted from statin‐treated HeLa S3 cells were applied to GST or GST‐GGTase‐III affinity columns. Bound proteins were eluted and incubated with recombinant GGTase‐III and BGPP. Reaction products were separated by SDS–PAGE, transferred to a nitrocellulose membrane, and probed with horseradish peroxidase (HRP)‐labeled avidin to detect biotinylated proteins. Mass spectrometry identified the 25 kDa protein as Ykt6.
-
DBiotin‐geranylation of recombinant Ykt6 by GGTase‐III. Bacterially produced recombinant untagged Ykt6 (left) was incubated with buffer, GGTase‐III, or RabGGTase in the absence or presence of BGPP for 30 min at 37°C. Biotin‐geranylated Ykt6 was detected as in (C).
Source data are available online for this figure.
