-
A
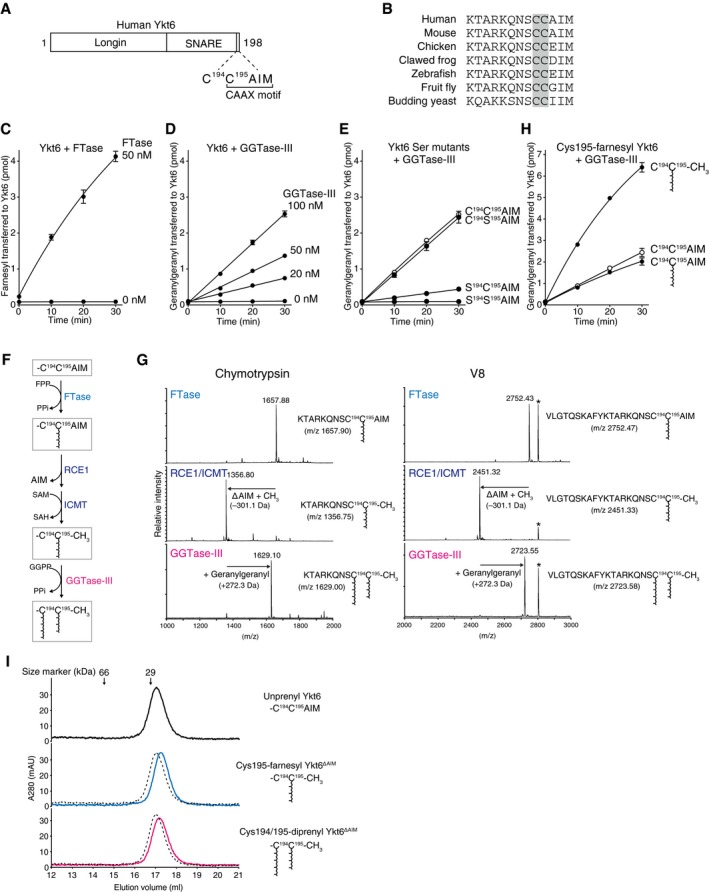
Schematic diagram showing the domain structure of human Ykt6 protein. Ykt6 has two cysteine residues, Cys194 and Cys195, at the fifth and fourth positions from the C‐terminus.
-
B
Alignment of the C‐terminal amino acid sequences of Ykt6 from various species. The conserved tandem cysteines are highlighted in gray.
-
C
Farnesylation of recombinant Ykt6 by FTase. Recombinant Ykt6 (5 μM) was incubated with FTase (0 or 50 nM) and 3H‐FPP (1 μM; ˜ 3,500 dpm/pmol) at 37°C. Reactions were stopped at the indicated time points, and the amount of 3H‐farnesyl transferred to Ykt6 was quantified by scintillation counting (mean ± SEM, n = 3).
-
D
Geranylgeranylation of recombinant Ykt6 by GGTase‐III. Recombinant Ykt6 (5 μM) was incubated with increasing concentrations of GGTase‐III (0–100 nM) and 3H‐GGPP (1 μM; ˜ 3,000 dpm/pmol) for the indicated times, and the amount of 3H‐geranylgeranyl transferred to Ykt6 was quantified (mean ± SEM, n = 3).
-
E
Geranylgeranylation of Cys to Ser mutants of Ykt6 by GGTase‐III. WT and mutant Ykt6 proteins (5 μM each) were incubated with GGTase‐III (100 nM) and 3H‐GGPP (1 μM) for the indicated times, and the amount of 3H‐geranylgeranyl transferred to Ykt6 was quantified (mean ± SEM, n = 3).
-
F
Sequential prenylation process of Ykt6. SAM, S‐adenosylmethionine; SAH, S‐adenosylhomocysteine; PPi, inorganic pyrophosphate.
-
G
Mass spectra of prenylated Ykt6 peptides. Recombinant Ykt6 was farnesylated by FTase (FTase), further processed by RCE1 and ICMT (RCE1/ICMT), and finally geranylgeranylated by GGTase‐III (GGTase‐III). After purification to homogeneity, the prenylated Ykt6 proteins were digested with chymotrypsin (left) or V8 protease (right) and analyzed by MALDI‐TOF mass spectrometry. The calculated m/z values for the C‐terminal peptide ions are shown in parentheses. The peaks labeled with an asterisk correspond to amino acids 14–38 of Ykt6 (AKVVLLKAAYDVSSFSFFQRSSVQE; m/z 2,806.48).
-
H
Effects of C‐terminal processing and Cys195 farnesylation on Cys194 geranylgeranylation by GGTase‐III. Purified recombinant unprenyl Ykt6, Cys195‐farnesyl Ykt6, and Cys195‐farnesyl Ykt6ΔAIM (5 μM each) were incubated with GGTase‐III (100 nM) and 3H‐GGPP (1 μM) for the indicated times, and the amount of 3H‐geranylgeranyl transferred to Ykt6 was quantified (mean ± SEM, n = 3).
-
I
Gel filtration chromatography of purified unprenyl Ykt6, Cys195‐farnesyl Ykt6ΔAIM, and Cys194/195‐diprenyl Ykt6ΔAIM (135 μg each) on a Superdex 200 column. Dotted lines indicate the elution profile of unprenyl Ykt6 for comparison. The prenylated Ykt6 proteins were eluted slightly later than the unprenylated Ykt6, suggesting more compact and stable folds.