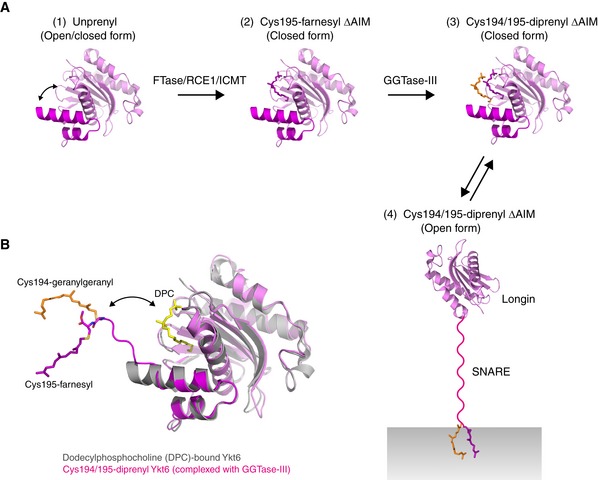
Figure EV4. Scheme of the double prenylation of Ykt6.
-
AModel for the sequential double prenylation of Ykt6. (1) Nascent unprenylated Ykt6 exists in both closed and open conformations. (2) Farnesylation of Cys195 stabilizes the closed conformation of Ykt6. (3) Doubly prenylated Ykt6 keeps the closed conformation by sequestering both farnesyl and geranylgeranyl groups into the putative prenyl binding groove. (4) Upon activation, the SNARE domain is unfolded and the C‐terminal two prenyl groups are inserted into the membrane.
-
BSuperposition of the dodecylphosphocholine (DPC)‐bound, closed form of Ykt6 (gray; PDB 3KYQ) and Cys194/195‐diprenyl Ykt6ΔAIM complexed to GGTase‐III (longin, pink; SNARE, magenta). GGTase‐III is omitted for clarity. The putative prenyl binding groove of Ykt6, occupied by DPC (yellow), is located closed to the active site of the enzyme. Upon binding to GGTase‐III, the Cys195‐linked farnesyl moiety accommodated in the putative prenyl binding groove of Ykt6 is translocated into the hydrophobic tunnel of the enzyme, allowing the transfer of geranylgeranyl moiety to Cys194. The attached two prenyl groups may easily translocate back to the prenyl binding groove of Ykt6.
