-
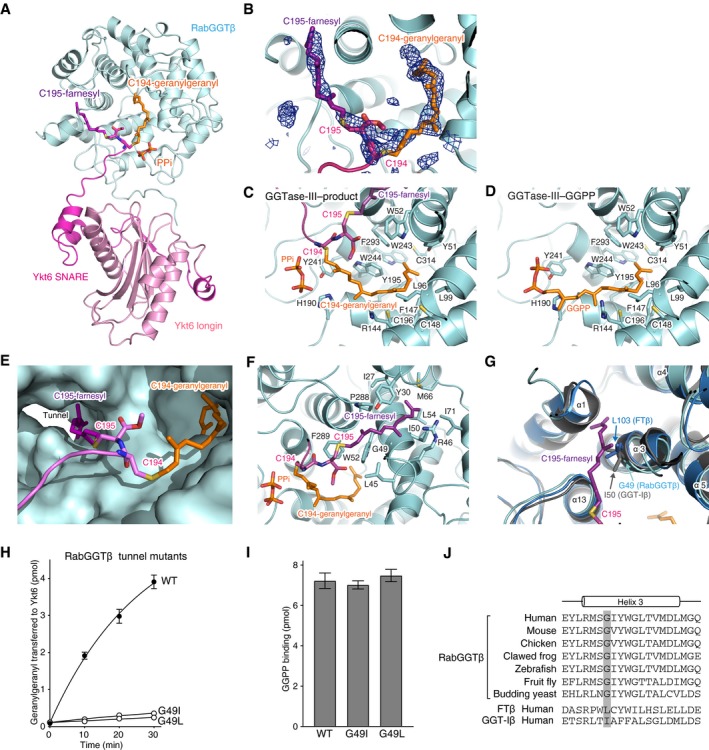
A
Overall view of the central cavity of the β subunit showing insertion of the C‐terminal tail of Ykt6. PTAR1 is omitted for clarity. The Cys194‐linked geranylgeranyl and Cys195‐linked farnesyl moieties are shown in orange and purple, respectively. PPi, inorganic pyrophosphate.
-
B
Electron density of the Cys194‐linked geranylgeranyl and Cys195‐linked farnesyl moieties (blue mesh; simulated‐annealing F
o–F
c omit map contoured at 3σ level).
-
C, D
Detailed views of the geranylgeranyl moiety in the GGTase‐III–product complex (C) and GGPP in the GGTase‐III–GGPP complex (D). Residues of the β subunit that form the lipid substrate binding pocket are shown.
-
E
Surface representation of the central cavity of the β subunit showing a tunnel formed near the active site. The Cys195‐linked farnesyl moiety is anchored into the hydrophobic tunnel.
-
F
Detailed view of the Cys195‐linked farnesyl group bound in the hydrophobic tunnel. Residues of the β subunit that line the hydrophobic tunnel are shown.
-
G
Superposition of the β subunits of FTase (blue), GGTase‐I (gray), and GGTase‐III (cyan). In FTase and GGTase‐I, the hydrophobic tunnel is blocked by the side chain of Leu103 and Ile50, respectively. The corresponding position is replaced by Gly49 in GGTase‐III.
-
H
Geranylgeranylation activity of WT GGTase‐III and tunnel mutants (100 nM each). Cys195‐farnesyl Ykt6ΔAIM (1 μM) and 3H‐GGPP (1 μM) were used as substrates (mean ± SEM, n = 3).
-
I
GGPP binding to GGTase‐III. WT GGTase‐III and tunnel mutants (1 μM each) were incubated with 3H‐GGPP (2 μM) at 4°C for 10 min. After desalting, enzyme‐bound 3H‐GGPP was quantified by scintillation counting (mean ± SEM, n = 3).
-
J
Alignment of the helix 3 sequences of RabGGTβ orthologues and human FTβ and GGT‐Iβ. The position of the conserved glycine residue (Gly49 in human) is highlighted in gray.