Figure 1.
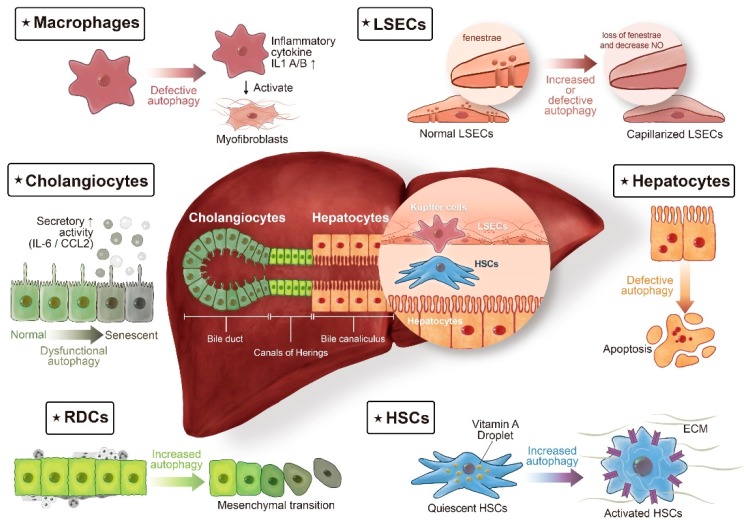
Autophagy in liver fibrosis: different cell populations tell different stories. The liver lobule consists of epithelial cells, hepatocytes, and cholangiocytes, and of non-parenchymal cells, such as macrophages (also termed Kupffer cells), liver sinusoidal endothelial cells (LSECs), and hepatic stellate cells (HSCs). In chronic liver diseases, extension of the fibrotic scars correlates with the presence of ductular reaction (DR). DR refers to extension of reactive ductular cells (RDCs), located in the Canals of Hering, the boundary line between hepatocytes and cholangiocytes. The cellular origin of RDCs is complicated, with different lines of evidence suggesting that in addition to being the progeny of hepatic progenitor cells, RDCs might also be derived from the proliferation of cholangiocytes or from ductular metaplasia of periportal hepatocytes. Autophagy, a complex regulatory pathway in liver fibrosis, exerts its profibrogenic effects in HSCs and RDCs. On the contrary, autophagy maintains cellular homeostasis in hepatocytes, cholangiocytes, macrophages, and LSECs, thereby counteracting fibrogenesis in the liver.