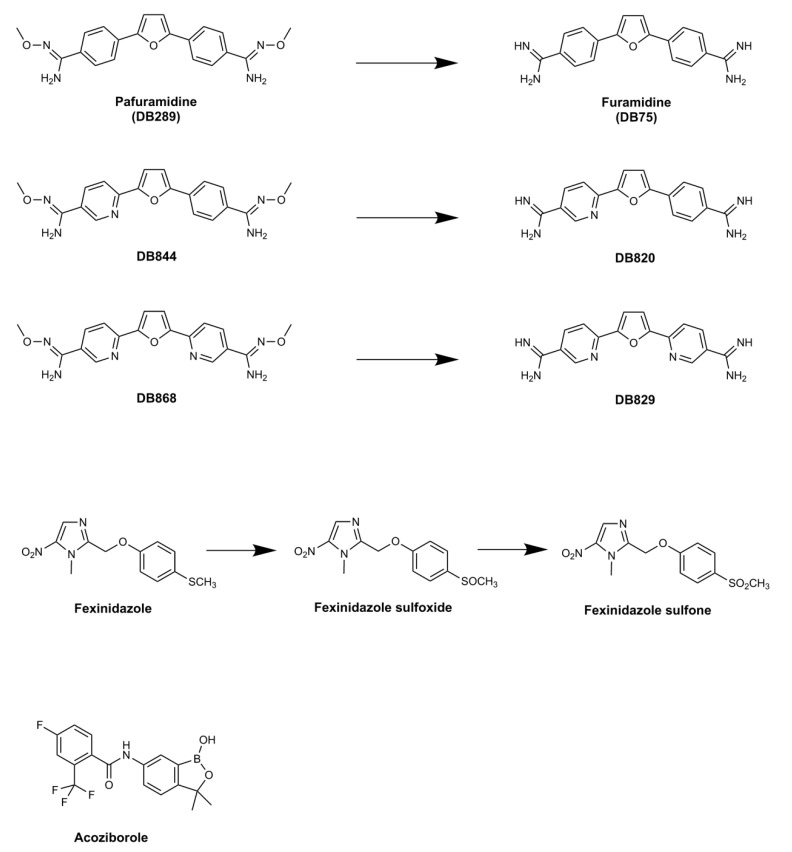
Figure 2.
Clinical trials with pafuramidine (DB289), which is the prodrug of furamidine (DB75), failed due to the appearance of renal toxicity during extended phase I safety profiling. The aza-derivatives including DB868—a prodrug of DB829, and DB844—a prodrug of DB820, also showed activity against stage 2 disease, but development was halted following identification of the toxicity associated with pafuramidine. Fexinidazole has been approved for use by the European Medicines Agency in 2018. The compound is converted to sulfoxide then sulfone derivatives after administration. Acoziborole is a benzoxaborole in clinical trials, where the efficacy of a single dosing of the drug as an oral medication against stage 2 disease is being assessed.