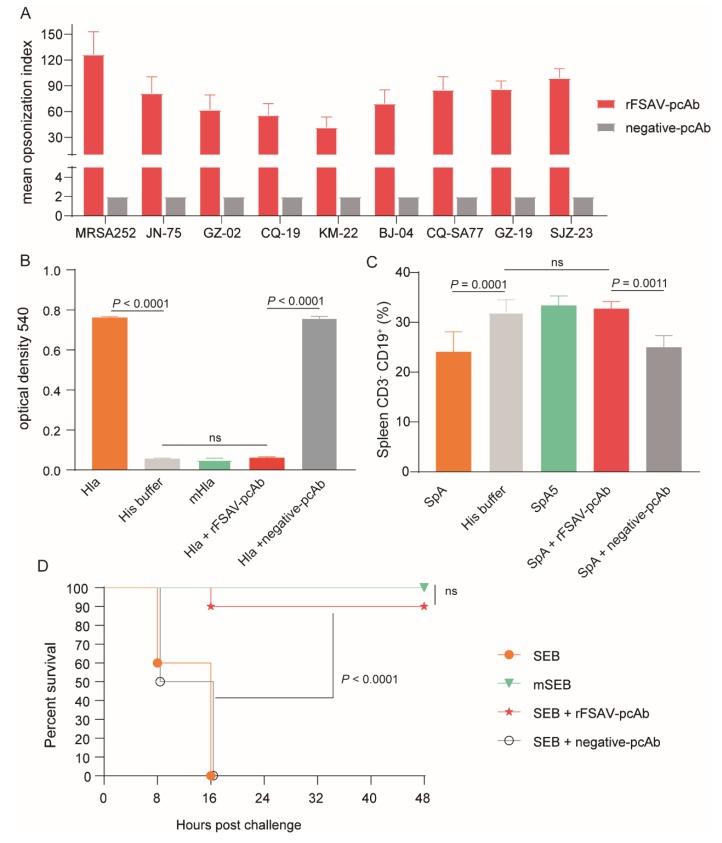
Figure 5.
Analysis of rFSAV-pcAb’s efficacy on promoting opsonophagocytosis and neutralizing toxins. (A) Comparative analysis of opsonophagocytic killing activity against different clinical isolations of S. aureus by rFSAV-pcAb. The mean opsonization index was calculated to determine killing activity. The data were shown as the mean ± SD derived from three independent experiments. (B–D) Hla, SpA or SEB were incubated with rFSAV-pcAb or negative-pcAb at 37 ℃ for 30 min prior to the start of the study, respectively. (B) Hemolytic activity assay. 1% Rabbit erythrocytes were incubated with Hla pre-incubated with rFSAV-pcAb or negative-pcAb at 37 ℃ for 30 min. The supernatant containing hemoglobin was measured spectrophotometrically at 540 nm to detect the hemolytic activity. The data were shown as the mean ± SD derived from three independent experiments. The p-values were calculated using one-way ANOVA (ns = no significance). (C) B cell superantigen activity assay. CD3− CD19+ B cells in splenic tissue of BALB/c mice (n = 5) treated with SpA pre-incubated with rFSAV-pcAb or negative-pcAb were quantified by FACS. The data were shown as the mean ± SD, and the p-values were calculated using one-way ANOVA. (D) Intestinal toxin activity assay. mice were injected i.p. with D-Galactosamine (200 mg/mL) followed by an intramuscular injection with SEB pre-incubated with rFSAV-pcAb or negative-pcAb. The survival rates were recorded every 8 h over a 2-day observation period post challenge. The Mantel-Cox log-rank test was used to calculate p-value.