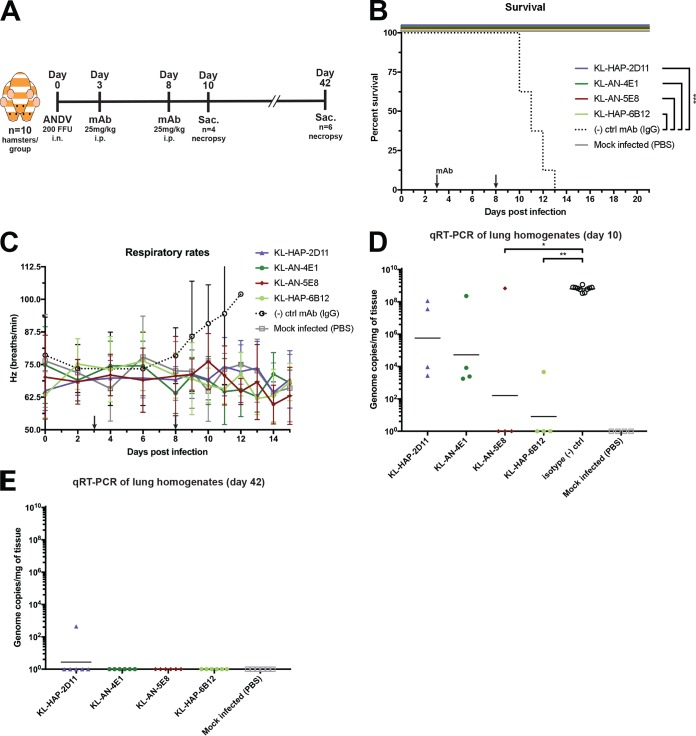
FIG 6.
In vivo studies in the Syrian hamster model of HCPS. (A) Syrian hamsters were inoculated intranasally (i.n.) with 200 FFU of ANDVCHI-9717869 or PBS (mock) and then injected i.p. with 25 mg/kg of MAb or PBS on day 3 and day 8 postinfection. Experimental overview, including necropsy endpoints. (B) Survival of the indicated treatment groups shown to day 21 (though all experimental groups survived until the predetermined survival endpoint of day 42). ***, P < 0.0001 via Mantel-Cox log-rank test. (C) Respiratory rates of each hamster depicted as an average/group. (D) Viral genome copies per milligram of homogenized lung tissue on day 10 postinfection. *, P = 0.0161; **, P = 0.0048 via Kruskal-Wallis test incorporating Dunn’s test for multiple comparisons. (E) Same as for panel D but on day 42 postinfection.