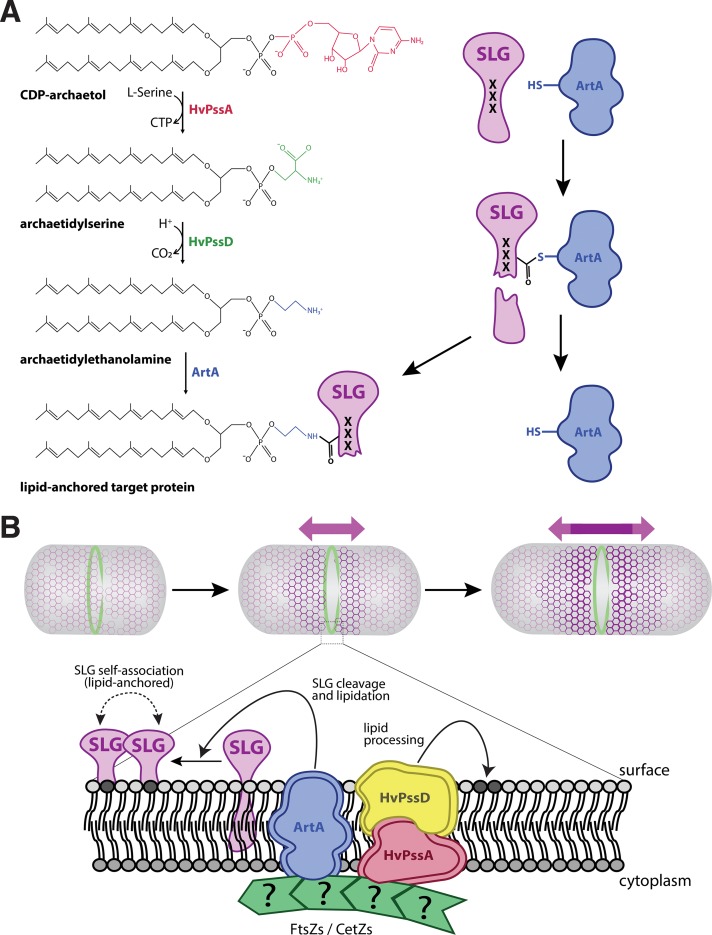
FIG 5.
A model for lipid attachment and cell growth involving HvPssA, HvPssD, and ArtA. (A) In our speculative model, CDP-archaeol is converted to archaetidylethanolamine in two steps involving HvPssA and HvPssD. ArtA acts as a peptidase and covalently links its active-site cysteine to a newly generated C terminus of its target protein, simultaneously releasing the C-terminal peptide. Then, the free amino group of ethanolamine attacks the thiocarboxylate, which marks the covalent attachment of the target protein to the ArtA active-site cysteine. This results in covalent attachment of the lipid to the C terminus of the target protein as a carboxamide, simultaneously releasing ArtA. The process of cleavage and lipidation is dependent on HvPssA and HvPssD, either by binding of archaetidylethanolamine to ArtA or by protein-protein interaction between ArtA and HvPssA or HvPssD. (B) Recruitment of ArtA, HvPssA, or HvPssD to the midcell promotes anchoring of surface proteins and insertion of new SLG into the S-layer at midcell, contributing to cell elongation and division.