FIG 3.
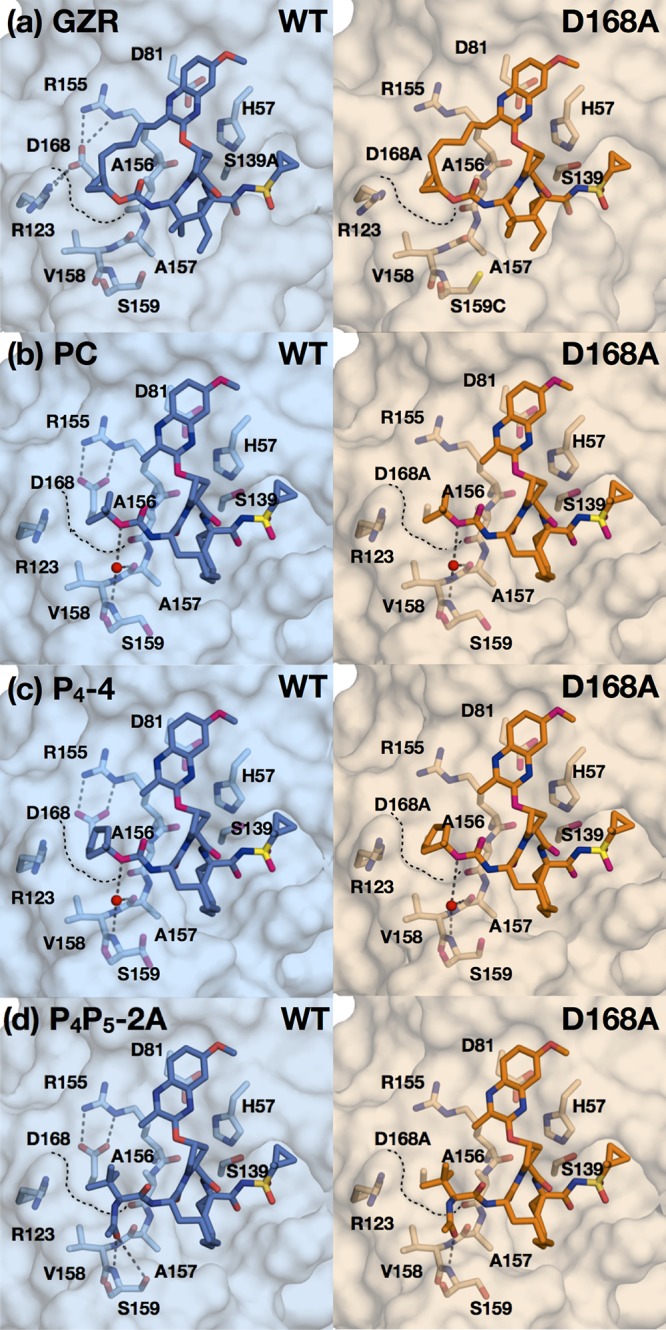
Binding of grazoprevir and designed PIs to WT and D168A protease active sites. Crystal structures of grazoprevir (GZR) (a), the parent compound (PC) (b), P4-4 (c), and P4P5-2A (d) bound to the wild-type and D168A proteases, as indicated. The protease active site is in surface representation, with the side chains of catalytic triad and S4 subsite residues shown as sticks. Water molecules are shown as nonbonded spheres (red), and hydrogen bonds (gray dashed lines) that stabilize S4 pocket side chains are displayed. Black dashed lines outline the surface of the S4 pocket where the D168A mutation is located.
