FIG 7.
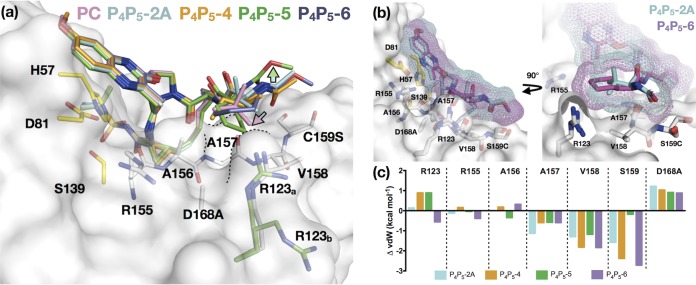
Binding mode of P4P5 inhibitors relative to that of the parent compound. (a) Superposition of cocrystal structures of the parent compound (PC), P4P5-2A, P4P5-4, P4P5-5, and P4P5-6, as indicated. The protease is in surface representation, and side chains of residues in and around the S4 pocket (white) and the catalytic triad (yellow) are displayed as sticks. The R123 can adopt two conformations (R123a and R123b). In all of the structures, R123 is in the commonly observed conformation (white; R123a) except for the P4P5-5 complex (green), where both conformations are observed (green). The contour of the S4 pocket is outlined in black dotted lines. The cyan-to-pink arrow indicates the displacement of tert-butyl group in the parent compound relative to that in P4P5-2A, and the green arrow shows a shift of the P5 extension away from the protease surface in P4P5-5 relative to that of the other compounds. (b) Superposition of P4P5-2A and P4P5-6 bound to D168A protease. The inhibitors are displayed as sticks with a mesh surface representation of the van der Waals surface. (c) Change in vdW contacts (ΔvdW) relative to those of the parent compound (PC) for P4P5-2A, P4P5-4, P4P5-5, and P4P5-6 with S4 residues.