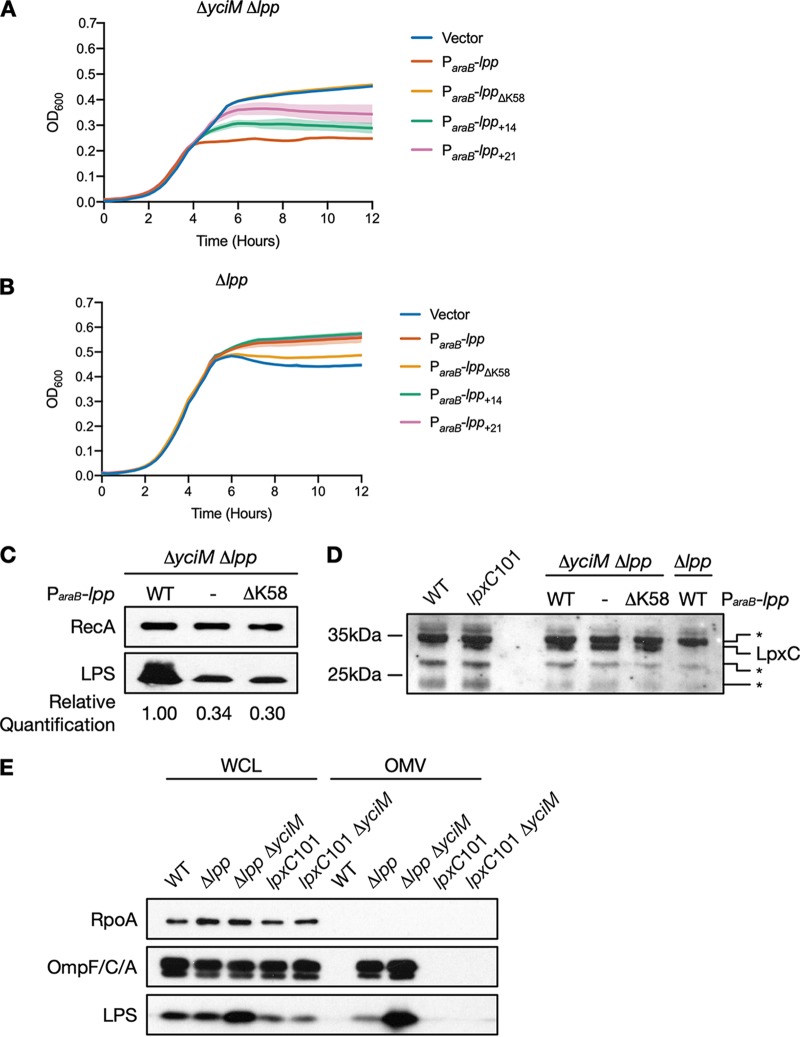
FIG 5.
Mutations in lpp suppress deletion of yciM by decreasing levels of LPS. (A and B) Growth of the ΔyciM Δlpp double mutant (A) and the Δlpp single mutant harboring plasmid pBAD18 or pBAD18 expressing wild-type lpp, lppΔK58, lpp+14, or lpp+21 from an arabinose-inducible promoter (B). Bacteria were diluted into LB supplemented with 0.5% arabinose. OD600, which was used as a proxy for growth, was measured every 15 min for 12 h. Data represent the means and standard deviations of three biological replicates. (C) Immunoblot analysis of LPS levels in the ΔyciM Δlpp double mutant containing pBAD18 or pBAD18 expressing wild-type lpp or lppΔK58 from an arabinose-inducible promoter. RecA was used as a loading control. (D) Immunoblot analysis of LpxC levels in the ΔyciM Δlpp double mutant containing pBAD18 or pBAD18 expressing wild-type lpp or lppΔK58 from an arabinose-inducible promoter. As LpxC levels are increased in the ΔyciM mutant (13, 16), the band corresponding to LpxC was identified by comparing ΔyciM Δlpp double mutants and a Δlpp single mutant expressing wild-type lpp from an arabinose-inducible promoter. This band was confirmed to be LpxC using the lpxC101 mutation, which significantly increases LpxC levels (29). The presence of a nonspecific band is indicated by an asterisk. (E) OM vesicles in the wild type, the Δlpp and lpxC101 single mutants, and the Δlpp ΔyciM and lpxC101 ΔyciM double mutants. RpoA was used as a cytoplasmic marker, while OmpC/F/A and LPS were used as markers of the OM. Increased amounts of OmpC/F/A and LPS in the OM vesicle fraction indicate that the Δlpp and Δlpp ΔyciM mutants hypervesiculate. Data in panels C to E are representative of two or more independent experiments. Lanes in panels C to E: −, vector; WT, wild type; WCL, whole-cell lysate; OMV, outer membrane vesicles.