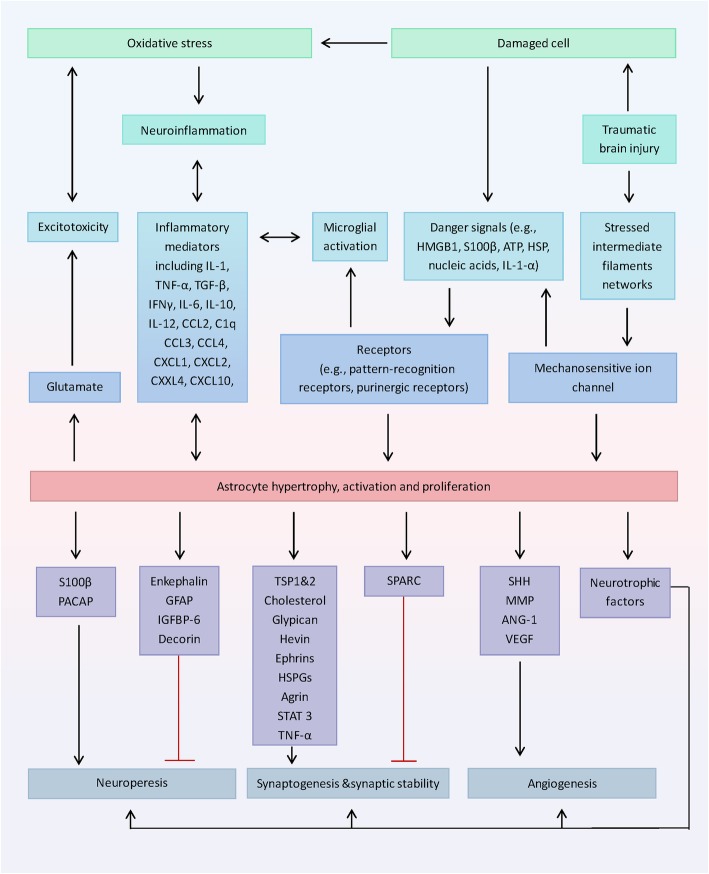
Fig. 3.
Potential therapeutic targets regarding astrocyte-derived molecules following TBI. Following TBI, damaged cells release danger signals. And stressed intermediate filaments networks within astrocytes activate ion influx through the mechanosensitive ion channel, resulting in the further release of danger signals. These signals serve to activate neuroglia and induce a robust sterile immune reaction and other secondary TBI pathogenesis. Reactive astrocytes secrete a wide range of factors that affect neurogenesis, synaptogenesis and synaptic stability, and angiogenesis, which may represent the therapeutic targets. Modulating the maladaptive microenvironment caused by neuroinflammation, excitotoxicity and oxidative stress post-TBI is also a considerable therapeutic strategy. ANG-1, angiopoietin-1; CCL, chemokine (C-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; GFAP, glial fibrillary acidic protein; HMGB1, high mobility group protein B1; HSP, heat shock proteins; HSPGs, heparan sulfate proteoglycans; IFN, interferon; IGFBP-6, insulin-like growth factor binding protein 6; IL, interleukin; MMP, matrix metalloprotein; PACAP, pituitary adenylate cyclase-activating peptide; SHH, sonic hedgehog; SPARC, secreted protein acidic and rich in cysteine; STAT3, signal transducer and activator of transcription-3; TBI, traumatic brain injury; TGF-β, transforming growth factor-β; TNF, tumor necrosis factor; TSP, thrombospondin; VEGF, vascular endothelial growth factor