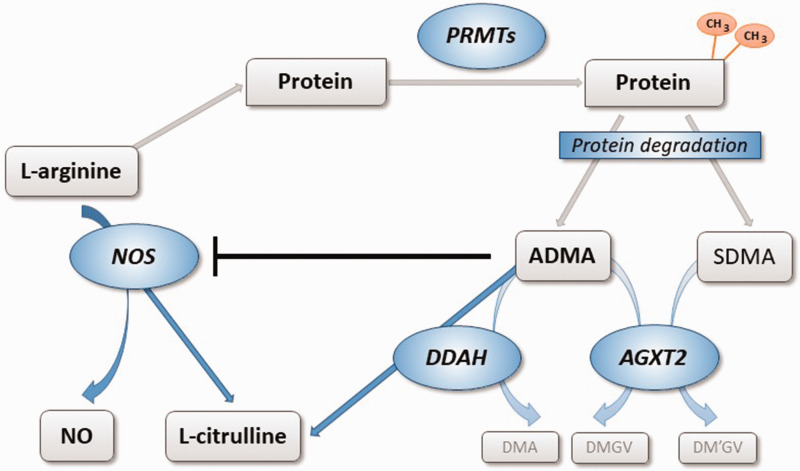
Fig. 1.
Biosynthesis and metabolism of ADMA and SDMA. L-arginine residues within specific proteins are subject to methylation by PRMTs. Degradation of di-methylated proteins during physiological protein turnover results in the release of ADMA and SDMA. ADMA is a competitive inhibitor of NOS, while SDMA does not directly impair NOS activity. ADMA, but not SDMA, is enzymatically degraded by DDAH into L-citrulline and DMA. DDAH exists in two distinct isoforms with different regulation and tissue distribution. Both dimethylarginines may be cleaved by an alternative pathway through the activity of AGXT2, resulting in the formation of symmetric or asymmetric dimethylguanidinovaleric acid (DMGV and DM'GV). AGXT2: alanine glyoxylate aminotransferase 2; ADMA: asymmetric dimethylarginine; PRMT: protein arginine N-methyltransferase; NO: nitric oxide; NOS: NO synthase; SDMA: symmetric dimethylarginine; DDAH: dimethylarginine dimethylaminohydrolase; DMA: dimethylamine.