FIGURE 19.14.
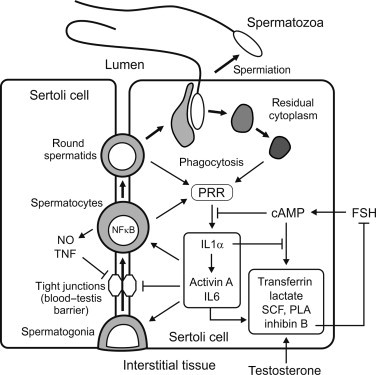
Hypothetical roles of inflammatory signaling pathways in control of spermatogenesis.
Spermatogonia enter meiosis to become spermatocytes and pass through the tight junctions between adjacent Sertoli cells. At the end of meiosis, the resulting haploid spermatids undergo major structural differentiation and are released from the seminiferous epithelium as spermatozoa (spermiation), leaving behind most their (residual) cytoplasm, which is phagocytosed by the Sertoli cells. Spermiation coincides with a surge of production of inflammatory mediators, such as interleukin-1α (IL1α), IL6, and activin A by the Sertoli cells, and tumor necrosis factor (TNF) and NO by the spermatogenic cells, which regulate the proliferation and maturation of the nearby spermatogonia and spermatocytes, and reorganization of the tight junctions to allow spermatocytes to pass into the luminal compartment. This localized inflammatory response may be mediated through activation of pattern-recognition receptors (PRR) in the Sertoli cell by endogenous spermatogenic molecules. Degenerating spermatogenic cells may also drive these pathways at other stages of the spermatogenic cycle, and inflammation caused by infection will disrupt these processes. Inflammatory mediators also regulate the supportive functions of the Sertoli cells, such as the production of transferrin, lactate, stem cell factor (SCF), plasminogen activator (PLA), and inhibin B, which are stimulated by androgens and by FSH acting via cAMP. Although these separate signaling pathways control some functional outcomes in common, there is clear evidence for reciprocal inhibitory regulation between the signaling pathways as well.
