eFigure 25-21.
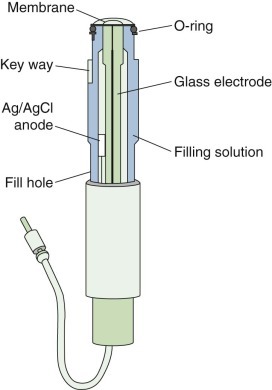
The structure of an oxygen electrode.
This electrode consists of platinum and silver electrodes placed in potassium chloride solutions, a polarizing voltage of 0.5 to 0.6 volts, and an electrolyte bridge to complete the circuit. Oxidation at the silver (Ag) electrode secondary to silver reacting with chloride ions to form silver chloride (AgCl) produces electrons that are consumed at the platinum electrode by reduction of oxygen. The flow of electrons (the current) is thus proportional to the concentration of oxygen at the platinum electrode.
