Abstract
Background
Coronavirus disease 2019 (COVID-19) is a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), first detected in China in December, 2019. In January, 2020, state, local, and federal public health agencies investigated the first case of COVID-19 in Illinois, USA.
Methods
Patients with confirmed COVID-19 were defined as those with a positive SARS-CoV-2 test. Contacts were people with exposure to a patient with COVID-19 on or after the patient's symptom onset date. Contacts underwent active symptom monitoring for 14 days following their last exposure. Contacts who developed fever, cough, or shortness of breath became persons under investigation and were tested for SARS-CoV-2. A convenience sample of 32 asymptomatic health-care personnel contacts were also tested.
Findings
Patient 1—a woman in her 60s—returned from China in mid-January, 2020. One week later, she was hospitalised with pneumonia and tested positive for SARS-CoV-2. Her husband (Patient 2) did not travel but had frequent close contact with his wife. He was admitted 8 days later and tested positive for SARS-CoV-2. Overall, 372 contacts of both cases were identified; 347 underwent active symptom monitoring, including 152 community contacts and 195 health-care personnel. Of monitored contacts, 43 became persons under investigation, in addition to Patient 2. These 43 persons under investigation and all 32 asymptomatic health-care personnel tested negative for SARS-CoV-2.
Interpretation
Person-to-person transmission of SARS-CoV-2 occurred between two people with prolonged, unprotected exposure while Patient 1 was symptomatic. Despite active symptom monitoring and testing of symptomatic and some asymptomatic contacts, no further transmission was detected.
Funding
None.
Introduction
In January, 2020, a novel virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the causative agent for a cluster of pneumonia cases initially detected in Wuhan City, Hubei province, China.1 SARS-CoV-2, which causes the disease now named coronavirus disease 2019 (COVID-19), had spread throughout China and to 26 additional countries as of Feb 18, 2020.2 Phylogenetic data implicate a zoonotic origin,3 and the rapid spread suggests ongoing person-to-person transmission. Several studies offer additional insight into person-to-person transmission.4, 5, 6, 7, 8, 9 However, substantial knowledge gaps remain regarding the transmissibility between humans, including the level of exposure to a confirmed case at which transmission is more likely to occur.
On Jan 23, 2020, Illinois, USA, reported the state's first laboratory-confirmed case (index case) of COVID-19 in a traveller who returned from Wuhan in mid-January, 2020. Subsequently, the first evidence of secondary transmission in the USA was reported on Jan 30, when the husband of the index patient, who had not travelled outside the USA, tested positive for SARS-CoV-2. Public health authorities did an intensive epidemiological investigation of the two confirmed cases.
This Article describes the first person-to-person transmission of COVID-19 in the USA, including the clinical and laboratory features of both patients and the assessment and monitoring of several hundred individuals with potential exposure to SARS-CoV-2.
Methods
Epidemiological investigation
The Illinois Department of Public Health, Chicago Department of Public Health, Cook County Department of Public Health, and DuPage County Health Department consulted with the US Centers for Disease Control and Prevention (CDC) for technical assistance and invited a CDC field team to assist with onsite investigations after laboratory confirmation of the first case of COVID-19.
Research in context.
Evidence before this study
We searched PubMed for articles published between database inception and Feb 18, 2020, describing transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using the search terms “severe acute respiratory syndrome coronavirus 2”, “SARS-CoV-2”, “novel coronavirus”, “2019-nCoV”, or “COVID-19”; and “transmission”, “person-to-person”, or “human-to-human”. We found 34 articles, of which 13 were primary reports of person-to-person transmission. None provided full details of the contact investigation and none were from North America.
Added value of this study
We detail prolonged, unprotected contact between a travel-related index case who was symptomatic and her husband, who subsequently acquired infection. This represents the first known person-to-person transmission of SARS-CoV-2 in the USA. We also detail a thorough contact investigation related to these cases. We identified, risk-stratified, and actively monitored almost 350 contacts of both cases. 43 contacts developed symptoms of fever, cough, or shortness of breath in the 14 days following their last exposure to either case and were tested for SARS-CoV-2, and 32 asymptomatic health-care professional contacts who had exposures across a range of risk levels were also tested for SARS-CoV-2. All 75 tested negative.
Implications of all the available evidence
Person-to-person transmission of SARS-CoV-2 occurred between two people with prolonged, unprotected exposure. No further transmission was detected, despite monitoring contacts for symptoms and testing all who developed fever, cough, or shortness of breath and testing a convenience sample of asymptomatic health-care professional contacts. Further detailed reports of contact investigations associated with cases of SARS-CoV-2 could improve understanding of the transmissibility of this novel virus.
Patients with COVID-19 were defined as individuals with laboratory-confirmed SARS-CoV-2 infection. Contacts were defined as people who reported or were identified to have potential exposure to a case on or after the day of symptom onset of the case (table 1 ). The earliest reported day with new symptoms was used as date of symptom onset. The date of symptom onset for the index case is considered day 0 for the purposes of this investigation, and all subsequent dates will be described by day of investigation (DOI), starting with DOI 0. In this Article, the numbers of contacts exposed to either case on or after the day of their first positive laboratory result are also presented.
Table 1.
Illinois risk classification of health-care personnel and community contacts with potential exposure to COVID-19
| Community contacts |
Health-care personnel contacts |
|||||
|---|---|---|---|---|---|---|
| Type of exposure | Example | Public health measure | Type of exposure | Example | Public health measure | |
| High-risk contacts | Living in the same household as, being an intimate partner of, or providing care in a non-health-care setting (such as a home) for a person with symptomatic laboratory-confirmed COVID-19 | Domestic partner | Home quarantine for 14 days after last exposure*; active symptom monitoring for 14 days after last exposure | Performing or being present in the room for a procedure likely to generate higher concentrations of respiratory secretions or aerosols while not using all recommended PPE†, or close contact while not wearing respiratory protection with a patient with laboratory-confirmed COVID-19 infection who was not wearing a facemask | Health-care personnel not wearing all recommended PPE who collected or were present for the collection of nasopharyngeal or oropharyngeal specimens‡ | Home quarantine*; exclude from work; active symptom monitoring for 14 days after last exposure |
| Medium-high-risk contacts | Prolonged or frequent contact with a person with symptomatic laboratory-confirmed COVID-19§ | Family members visited for prolonged periods or close work associates | Home quarantine for 14 days after last exposure*; active symptom monitoring for 14 days after last exposure | Prolonged (15 min or more) contact with a patient with laboratory-confirmed COVID-19 infection or their secretions or excretions while not using all recommended PPE† | Performing a check of the vital signs and phlebotomy on a masked patient while wearing gloves and a surgical mask | Exclude from work; active symptom monitoring for 14 days after last exposure |
| Medium-risk contacts | Close contact with a person with symptomatic laboratory-confirmed COVID-19 and not having any exposures that meet a high-risk or medium-high-risk definition | Colleagues who work less closely together but still have regular face-to-face contact | Active symptom monitoring for 14 days after last exposure | More than brief contact (>1–2 min) with a patient with laboratory-confirmed COVID-19 infection or their secretions or excretions while not using all recommended PPE† that does not meet a high-risk or medium-high-risk definition | Examined patient for 5 min while wearing mask, gown, gloves, and faceshield (but no respirator) | Exclude from work; active symptom monitoring for 14 days after last exposure |
| Low-risk contacts | Being in the same indoor environment with (or within 2 h of) a person with symptomatic laboratory-confirmed COVID-19 | Shared a hospital or outpatient waiting room or entered space within 2 h of a case | Active symptom monitoring for 14 days after last exposure | Any duration of contact with a patient with laboratory-confirmed COVID-19 while using all recommended PPE†, brief interaction with the patient (1–2 min) not involving direct contact while not using all recommended PPE†, or working at the same time and location as a confirmed case but unsure whether they were in the same room | Examined patient while wearing gloves, gown, faceshield, or goggles and appropriate, fit-tested respiratory protection; entered patient’s room briefly to bring the patient a drink but did not have direct contact with the patient or their secretions or excretions | Active symptom monitoring for 14 days after last exposure |
| Non-contacts | Interactions with a person with symptomatic laboratory-confirmed COVID-19 that do not meet high-risk, medium-high-risk, medium-risk, or low-risk conditions | Walking by a patient in a corridor | None | Did not meet any of the high-risk, medium-high-risk, medium-risk, or low-risk conditions | Walking by a patient in a corridor | None |
COVID-19=coronavirus disease 2019. PPE=personal protective equipment. CDC=US Centers for Disease Control and Prevention. MERS-CoV=Middle East respiratory syndrome coronavirus.
Implemented after identification of the second case of laboratory-confirmed COVID-19 in Illinois on Jan 30, 2020.
Recommended PPE includes respiratory protection (ie, respirator), goggles or faceshield that covers the front and sides of face, gloves, and a gown.
Risk categorisation was developed on Jan 26, 2020, before published guidance from CDC for COVID-19.10 Criteria were based on published MERS-CoV guidance and additional input from CDC subject matter experts. Close contact was defined as being within approximately 6 feet or within the room or care area of a confirmed COVID-19 case (including sharing a health-care waiting area or room), or being in a shared air space vacated by a confirmed case within the previous 2 h. Transient interactions, such as walking by confirmed case, were not considered close contact. Of note, nasopharyngeal and oropharyngeal specimen collection were not listed as aerosol-generating procedures in the CDC guidance, but were included as high-risk exposures in this investigation.
Risk categorisation was developed on Jan 31, 2020, before published guidance from CDC for COVID-19.11 Criteria were based on published MERS-CoV guidance and additional input from CDC and state and local health officials. The medium-high-risk classification was included owing to the identification of some community contacts who did not meet the highest category of exposure risk but were nevertheless concerning.
Patients with COVID-19 were interviewed using a standardised questionnaire to identify symptom history, locations visited while symptomatic, and individuals with whom they had contact while symptomatic. The Illinois COVID-19 Investigation Team, comprised of local and state public health staff and the CDC field team, worked with locations visited (eg, workplaces, retail establishments, or health-care facilities) by patients with COVID-19 to identify additional individuals who might have had exposures to SARS-CoV-2. To identify possible exposures in health-care personnel, patient logs and staffing records were obtained and reviewed for all health-care settings visited by patients with COVID-19. Security footage was reviewed to identify additional health-care personnel and patients who had contact with patients with COVID-19 during transport through the admitting hospital. Health-care personnel were defined as all people working in health-care settings who had the potential for exposure to infectious materials,12 including members of the Illinois COVID-19 Investigation Team. All other contacts were classified as community members, including patients in the same indoor environment in a health-care setting (eg, a hospital waiting room).
Exposure risk classification
Health-care personnel and community members with potential exposure to SARS-CoV-2 were interviewed using standardised contact questionnaires to assess exposure and whether the individual had true contact with a patient with COVID-19. Exposure risk was classified according to frameworks designed by members of the Illinois COVID-19 Investigation Team in consultation with CDC subject-matter experts (table 1). These frameworks were based on published guidance for Middle East respiratory syndrome coronavirus and designed and implemented before interim risk assessment guidance for COVID-19 released by CDC.10, 11
Active monitoring of contacts
All health-care personnel and community contacts assessed to have had low-risk, medium-risk, medium-high-risk, or high-risk exposures were enrolled in active symptom monitoring, which continued for 14 days after last exposure to a patient with COVID-19. Active symptom monitoring was done using Research Electronic Data Capture software (Vanderbilt University, Nashville, TN). Contacts received automated, twice-daily emails inquiring about symptoms, including cough and shortness of breath, and a request for a self-measured temperature. If symptoms or fever (temperature of >38°C) were reported, or if contacts did not respond or declined email monitoring, public health officials telephoned contacts daily. For hospital-based health-care personnel not excluded from work, pre-shift symptom assessment for fever, cough, or shortness of breath was implemented by hospital occupational health services. To identify any contacts (including those that could not be reached for active symptom monitoring) seeking care for fever, cough, or shortness of breath at an emergency department, the Illinois Department of Public Health used locally available, near real-time surveillance data received from regional acute care hospitals, which included symptom and diagnoses data and personally identifiable information for matching.
If a contact developed fever, cough, or shortness of breath during active symptom monitoring, they were classified as a person under investigation (PUI; a standard case designation used by CDC during an outbreak)13 and were isolated and tested for SARS-CoV-2.
Specimen collection and laboratory testing
For PUIs, specimens were collected and sent to CDC for testing. Specimens included upper (nasopharyngeal and oropharyngeal swabs) and lower respiratory specimens (sputum) if spontaneously produced. For patients with COVID-19, nasopharyngeal, oropharyngeal, serum, sputum, urine, and stool specimens were collected and sent to CDC for testing at initial presentation, and then every 2–3 days. Additionally, a convenience sample of 32 asymptomatic health-care personnel contacts had one-time nasopharyngeal and oropharyngeal specimens obtained at least 7 days from their highest-risk exposure. All health-care personnel contacts were offered testing, but laboratory capacity and availability of health-care personnel to undergo testing were limited in the setting of this urgent investigation. Before Patient 2 reported symptoms to public health investigators, nasopharyngeal and oropharyngeal swabs were also collected from Patient 2 owing to his high-risk exposures to Patient 1.
Specimens were collected per CDC guidance.14 All specimens were refrigerated at 2–8°C before shipping on icepacks to CDC. CDC did real-time RT-PCR (rtPCR) to detect three separate genetic markers of SARS-CoV-2, as previously described.15 The cycle threshold value ranges for the three markers were interpreted as a semi-quantitative measure of the RNA concentration in the specimen.
Role of the funding source
There was no funding source for this study.
Results
Patient 1 is a female in her 60s who travelled to Wuhan on Dec 25, 2019, and returned to Illinois on Jan 13, 2020, and who was not symptomatic while travelling. In Wuhan, she visited a hospitalised relative regularly and visited other family members who had undiagnosed respiratory illnesses, one of whom was later hospitalised with viral pneumonia. No contacts had laboratory-confirmed COVID-19, but it is unknown whether any were tested for SARS-CoV-2.
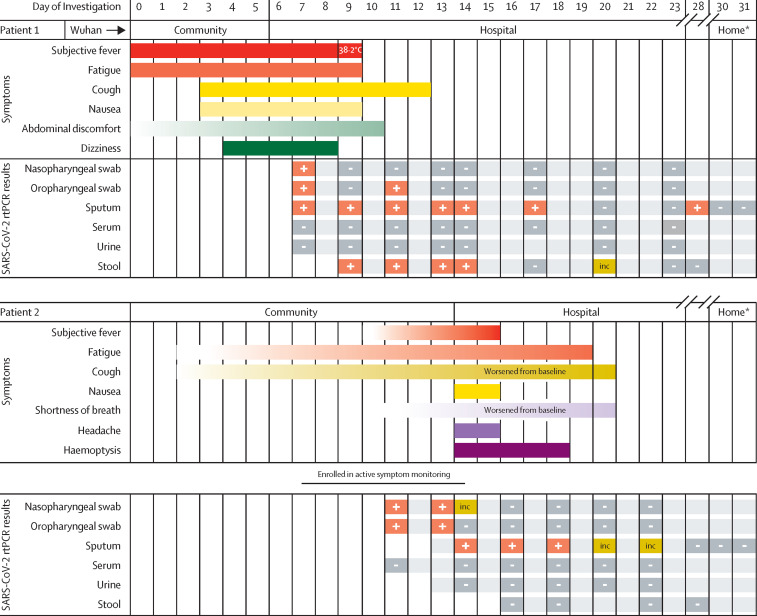
On DOI 6, she sought care at an outpatient clinic for fever, fatigue, and cough and was hospitalised that day for pneumonia. She was reported to public health authorities as a PUI on DOI 7. Retrospectively, she reported that her symptoms, which also included nausea, abdominal discomfort, and dizziness, started as early as 6 days before admission (figure ).
Figure.
Symptoms and results of rtPCR testing for SARS-CoV-2 by day of investigation
Gradient shading indicates unclear period of symptom onset from patient report. inc=inconclusive result. rtPCR=real-time RT-PCR. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *Patient 1 and Patient 2 in home isolation.
Before hospitalisation, she had frequent, close contact with her husband on DOI 0–6 when she had an active cough. Her husband had not travelled to Wuhan. She and her husband live together, eat together, share a bed, and have frequent face-to-face interactions. Facemasks or other personal protective equipment (PPE) were not used at the home. Her husband was classified as having high-risk exposures and began active symptom monitoring on DOI 7 with specimen collection on DOI 11, before his report of any new symptoms.
Patient 2 has chronic obstructive pulmonary disease, with a chronic, productive cough and baseline dyspnoea; therefore, the timing of symptom onset related to COVID-19 was difficult to determine (figure). When first interviewed as a contact on DOI 7, he reported no fever or change in chronic respiratory symptoms. Later, he reported increased dyspnoea and sputum production starting on DOI 11, which was also the first day of specimen collection as a contact in Patient 1's investigation. Upon further interview of Patient 2's contacts, it was suggested that some non-specific symptoms might have started as early as DOI 3, with fatigue and worsening cough. On DOI 14, he reported new haemoptysis and worsening dyspnoea through active monitoring. He was promptly admitted to the hospital and placed in an airborne infection isolation room (AIIR). Nasopharyngeal and oropharyngeal specimens from DOI 11 tested positive for SARS-CoV-2 on DOI 15.
On hospital admission, vital signs, and physical examination for Patient 1 were within normal limits. Her chest radiograph demonstrated no abnormalities, but a CT scan of her chest revealed bilateral multifocal infiltrates and mediastinal and hilar lymphadenopathy. On admission, Patient 2 had mild tachypnoea and coarse breath sounds with mild wheezes bilaterally, although whether these signs represented a change from his baseline status is unclear. Patient 2's chest radiograph showed emphysematous changes and right lower lobe infiltrates consistent with pneumonia. For both patients, testing for other viral and bacterial respiratory infections was negative. Both experienced mild leukopenia (Patient 1 white blood count nadir 3·0 × 103 cells per μL, Patient 2 nadir 3·4 × 103 cells per μL), lymphopenia (Patient 1 absolute lymphocyte count nadir 0·7 × 103 cells per μL, Patient 2 nadir 0·8 × 103 cells per μL), and mild elevations in aspartate aminotransferase and alanine aminotransferase (Patient 1 peak 46 units per L and 66 units per L, Patient 2 peak 47 units per L and 75 units per L). No other remarkable laboratory results were noted.
Both patients recovered and were discharged to home isolation on DOI 23. Hospital admission was extended while arrangements were made for home isolation. Home isolation for both patients was lifted on DOI 33, following two sets of negative respiratory specimens collected 24 h apart.
Patient 1 wore a facemask in the emergency department waiting room and was placed on droplet precautions in the emergency department and for the first 10 h after admission. She was subsequently transferred to an AIIR, where health-care personnel entering the patient's room were required to adhere to Standard, Contact, and Airborne Precautions, including hand hygiene, gloves, gown, respirator, and eye protection.16 Health-care personnel were enrolled in active monitoring, and potential breaches were recorded and investigated to determine risk classification. Patient 2 was immediately evaluated and admitted to an AIIR and placed on Transmission-Based Precautions as described for Patient 1.
For Patient 1, initial nasopharyngeal, oropharyngeal, and sputum specimens collected on DOI 7 were positive, whereas serum and urine were negative. Her initial sputum rtPCR cycle threshold values ranged between 24–25, indicating high viral burden before isolation. Sputum specimens remained positive longer than all other specimens for both cases (figure, appendix pp 3–4). Stool specimens collected for Patient 1 also remained positive longer than nasopharyngeal and oropharyngeal specimens; however, Patient 2 had no positive stool specimens. Neither Patient 1 or 2 had serum or urine specimens that tested positive for SARS-CoV-2.
372 contacts of either Patient 1 or Patient 2 were identified. Public health investigators were able to assess exposure risk and actively monitor symptoms for 347 (93%) of the 372 contacts, including 222 (94%) of 236 contacts with exposure on or after the date of first positive specimen collection. There were 25 people that had insufficient contact information to complete active monitoring. None of these individuals were found to have emergency department visits with fever, cough, or shortness of breath using near real-time surveillance data received from regional acute care hospitals for 14 days after their last exposure. Data presented are for those actively monitored. Of these 347 contacts, 195 (56%) were health-care personnel and 152 (44%) were community members. Although the majority of monitored contacts (228 [66%] of 347) had low-risk exposures, 119 (34%) had exposures of medium risk or greater (table 2 ).
Table 2.
Actively monitored contacts and PUIs owing to contact with a patient with COVID-19, Illinois, USA, 2020
| Since first reported date of symptom onset |
On or after date of first positive specimen |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total contacts | Did not become a PUI | Met PUI criteria* | PUIs positive for COVID-19† | Total contacts | Did not become a PUI | Met PUI criteria* | PUIs positive for COVID-19† | ||
| Community contacts | |||||||||
| High risk | 1 | 0 | 1 | 1/1 | 1 | 0 | 1 | 1/1 | |
| Medium high | 7 | 5 | 2 | 0/2 | 1 | 1 | 0 | ·· | |
| Medium | 28 | 24 | 4 | 0/4 | 0 | 0 | 0 | ·· | |
| Low | 116 | 111 | 5 | 0/5 | 65 | 61 | 4 | 0/4 | |
| Total | 152 | 140 | 12 | 1/12 | 67 | 62 | 5 | 1/5 | |
| Health-care personnel contacts | |||||||||
| High risk | 32 | 28 | 4 | 0/4 | 22 | 20 | 2 | 0/2 | |
| Medium high | 39 | 30 | 9 | 0/9 | 29 | 24 | 5 | 0/5 | |
| Medium | 12 | 6 | 6 | 0/6 | 9 | 5 | 4 | 0/4 | |
| Low | 112 | 99 | 13 | 0/13 | 95 | 84 | 11 | 0/11 | |
| Total | 195 | 163 | 32 | 0/32 | 155 | 133 | 22 | 0/22 | |
| Total contacts | 347 | 303 | 44 | 1‡ | 222 | 195 | 27 | 1‡ | |
Data are n or n/N. PUI=person under investigation. COVID-19=coronavirus disease 2019.
US Centers for Disease Control and Prevention PUI criteria for contacts of a confirmed case: fever (subjective or objective) or signs or symptoms of lower respiratory illness (eg, cough or shortness of breath).
PUIs were tested for COVID-19 using real-time RT-PCR for severe acute respiratory syndrome coronavirus 2. Only results from PUIs tested for COVID-19 in this investigation are presented here.
The index patient, Patient 1, is excluded from this total
Although Patient 1 and 2 live together and were hospitalised in the same facility, and therefore shared several common contacts (65 shared community contacts from emergency department or outpatient waiting rooms and 28 health-care personnel who interacted with both patients), they also had many unique contacts. Patient 1 had 92 unique health-care personnel contacts and 16 unique community contacts, including one household contact (Patient 2). Patient 2 had 75 unique health-care personnel contacts and 71 unique community contacts, including 51 from outpatient waiting rooms.
The majority of contacts (303 [87%] of 347 total monitored contacts and 195 [88%] of 222 monitored contacts on or after the date of first positive specimen collection) did not develop symptoms consistent with PUI criteria. Additionally, surveillance data from Illinois acute care hospitals indicated that no asymptomatic monitored contacts or other contacts who could not be reached for active symptom monitoring presented to an emergency department with fever, cough, or shortness of breath during DOI 6–30.
During active symptom monitoring, 44 (13%) of 347 total contacts became PUIs, including 27 (12%) of 222 monitored contacts who had exposures on or after the date of first positive specimen collection.
As a household contact, Patient 2 was the only community member who had a high-risk exposure. He became a PUI and subsequently the only other patient with COVID-19 in this investigation. Of the remaining 43 PUIs, all tested negative for SARS-CoV-2 while symptomatic; 32 of these PUIs were health-care personnel and 11 were community contacts. Although 18 (41%) of 44 PUIs had low-risk exposures, 26 (59%) had exposures of medium risk or greater.
32 health-care personnel contacts who were not PUIs had one-time nasopharyngeal and oropharyngeal specimens collected 7–14 days after their highest-risk exposure. All of these exposures occurred on or after the date of first positive specimen collection of a patient with COVID-19. 21 (66%) of these asymptomatic health-care personnel had exposures of medium risk or greater. All were negative for SARS-CoV-2 at the time of testing.
Discussion
This Article documents the first known person-to-person transmission of SARS-CoV-2 in the USA. Transmission occurred between close household contacts, from an index travel-associated case who subsequently transmitted the infection to her husband. Their prolonged, unprotected close contact occurred across multiple days early in her illness, before Patient 1 sought clinical care. No additional cases of COVID-19 were identified through active symptom monitoring of several hundred community and health-care personnel contacts, testing of symptomatic PUIs, or screening of a subset of asymptomatic health-care personnel contacts. These data suggest that person-to-person transmission of COVID-19 might be most likely to occur through unprotected, prolonged exposure to a patient with symptomatic COVID-19. Our experience of limited transmission of SARS-CoV-2 differs from that documented in Wuhan, where transmission has been reported to occur across the wider community and in health-care personnel,6 and from experiences of other similar coronaviruses.17, 18, 19 The severity of illness, the extent of viral shedding, and timing of exposures to a symptomatic patient might all have contributed to the limited transmission described here. Infection control measures within the hospital setting and an aggressive public health response might also have prevented further exposures.
Much like the first US case of COVID-19 in Washington,20 both Illinois patients had mild-to-moderate illnesses that started with non-specific symptoms, making early identification difficult for patients, clinicians, and public health investigators. Furthermore, Patient 2's baseline cough and dyspnoea made identifying new symptoms challenging. These factors have implications for detection of future cases. Clinicians and public health officials should maintain a low threshold for testing in patients with comorbidities that might obscure obvious signs and symptoms of COVID-19.
The timing and duration of viral shedding after SARS-CoV-2 infection is unknown. In the two Illinois patients, sputum specimens remained rtPCR-positive longer than other specimen types. Recognising that rtPCR testing detects any SARS-CoV-2 RNA, not necessarily infectious virus, further studies are needed to understand how viral shedding and detection are associated with transmission. Such studies have implications for public health recommendations regarding the type and duration of isolation required for patients with COVID-19 and will allow for more focused and targeted contact tracing and testing of appropriate specimens based on duration of illness.
These data are preliminary and subject to several limitations. First, this Article describes only one known transmission event and the associated contact investigation. Findings might not be generalisable or representative of broader transmission patterns. Second, this investigation might not have identified all individuals with potential exposure to SARS-CoV-2, because epidemiological investigations are dependent on individuals' recall of places visited, people seen, and symptom onset. The date of symptom onset for Patient 2 was especially difficult to ascertain. Given this uncertainty, we applied a conservative approach for identifying contacts of Patient 2 by using the earliest reported date of possible symptom onset, DOI 3. This could have artificially increased the number of contacts and provided false reassurance of infrequent transmission. Therefore, we also present data separately for exposures that occurred on or after the first known date of viral positivity.
Third, this investigation took place before published CDC guidance for classifying exposure risk among contacts of patients with COVID-19.10, 12 The risk classification used here differed from the now published guidance in some key areas. For example, we considered nasopharyngeal and oropharyngeal specimen collection aerosol-generating procedures, and therefore classified health-care personnel performing these without all recommended PPE as high risk, whereas they are classified as medium risk according to the guidance. Additionally, we included community members as contacts if they entered the same indoor environment (eg, hospital waiting room) within 2 h of a patient with COVID-19, an approach based on other viruses with airborne transmission patterns, such as measles. Current interim guidance requires contacts to have been in the room at the same time as a patient with COVID-19. Therefore, the risk stratifications used here might not be comparable to future investigations using this guidance.
Fourth, nasopharyngeal and oropharyngeal specimens collected on both PUIs and asymptomatic health-care personnel contacts were collected at a single timepoint; a single negative SARS-CoV-2 rtPCR might not be sufficient to definitively rule out infection over a 14-day incubation period, and only a convenience sample of a minority of health-care personnel contacts were tested in this study, albeit weighted to capture those with higher-risk exposures. Additionally, the active symptom monitoring employed here would not detect asymptomatic transmission. Future serological studies of exposed contacts will allow a better understanding of asymptomatic infection rates. Furthermore, updated CDC guidance recommends including sore throat as a possible symptom of COVID-19 when evaluating health-care personnel,10 whereas in this investigation, only those with fever, cough, or shortness of breath were tested for SARS-CoV-2.
Nevertheless, our ongoing investigation has only detected transmission of SARS-CoV-2 in a single household contact with frequent, prolonged interactions with the index patient. The absence of COVID-19 among health-care personnel supports recommendations regarding appropriate infection control. These findings also support CDC's assessment that, without using appropriate PPE, people living in the same household as, or providing care in a non-health-care setting for, a person with symptomatic laboratory-confirmed COVID-19 have high-risk exposure.21 In these contexts, CDC's recommendation for people with high-risk exposures to remain quarantined with no public activities might be effective in reducing onward person-to-person transmission of SARS-CoV-2.11 Given the difficulty in detecting new symptoms in patients with underlying lung disease, CDC recommends that clinicians considering a diagnosis of COVID-19 should discuss testing with public health departments on a case-by-case basis.22 Patients with potential exposure to SARS-CoV-2 with a fever, cough, or shortness of breath should call their health-care provider before seeking care so that appropriate preventive actions can be implemented.21 Health-care facilities should rapidly triage and isolate suspected PUIs and notify infection prevention services and local health departments for support in testing, management, and containment efforts.22
Acknowledgments
Acknowledgments
We thank the patients, staff at local and state health departments of Illinois, staff at the US Centers for Disease Control and Prevention (CDC) Division of Viral Disease Laboratory, CDC staff at the Emergency Operations Center, and members of the COVID-19 response teams at the local, state, and national levels for their input and collaboration on this investigation. For their partnership and dedication, we thank the clinical team and associates of AMITA Health St Alexius Medical Center, including Charmaine Arosen, Roxann Barber, Candi Boros, Jeffrey Butler, Joan Cappelletti, Carla Casia, James Collier, Paula Crossen, Polly Davenport, Steven Dlugo Mindy Doumani, Suzanne Dwyer, Allison Folkerts, Darlene Gallagher, Karen Gorman, Melissa Granato, Michael Handler, Michelle Hereford, Lauren Johnson, Michelle Johnston, Lynwood Jones, Mary Kerber, Kihe Kim, Craig Kuhl, Monica Kziazcyk, Adam Leung, Cindie Lietzke, Ann Lucey, Stuart Marcus, Tim Mathews, Rosemarie Mayer-Semar, Connie Noltemeyer, Shawn O'Connor, Mary Ann Palermo, Ana Payne, Carol Pfeifer, Chris Quinlan, Monica Rodriguez-Simzky, Deborah Rudd, Johanna Senyk, Vrusha Shastri, Natalie Sowizral, Lisa Sturm, Jeremy Swaw, Thor Thordarson, Jennylee Vazquez, Kim Vogt, Jaime Zalewski, and Eric Zemaitaitis.
The opinions expressed by authors contributing to this Article do not necessarily reflect the opinions of the CDC or the institutions with which the authors are affiliated.
Contributors
IG, TDM, JCH, and HLK each led aspects of the contact investigation and JRV and JEL provided overall leadership and guidance to the investigation. IG, TDM, JCH, HLK, DC, KJ, RR, SM-E, SRB, MP, MJF, RKC, KAW, DPB, HER, MW, CW, DM, JaK, SAN, IB, MWJ, VSD, MTP, JuK, EMC, NOE, JRV, and JEL completed the investigation of cases and collected epidemiological data. NSA, WCS, and NFH provided clinical care to the patients and assisted with clinical descriptions. XL and SL described and did laboratory specimen processes and testing for all patients. JCH, HLK, SAN, IB, VC, CMM, MAR, SIG, and JRV provided technical assistance and input in content areas, including infection control, epidemiological methods, medical countermeasures, and subject matter expertise. IG, TDM, and JEL drafted and revised this manuscript. All authors reviewed, revised, and approved the final manuscript.
Declaration of interests
We declare no competing interests.
Contributor Information
Illinois COVID-19 Investigation Team:
Sarah Brister, Kristin Goldesberry, Stacey Hoferka, Dejan Jovanov, Dawn Nims, Lori Saathoff-Huber, Chantel Hoskin Snelling, Hira Adil, Raabiah Ali, Elaina Andreychak, Kelley Bemis, Mabel Frias, Pearl Quartey-Kumapley, Kristin Baskerville, Elizabeth Murphy, Emily Murskyj, Zach Noffsinger, Janice Vercillo, Apryll Elliott, Uche S. Onwuta, Danielle Burck, Glen Abedi, Rachel M. Burke, Ryan Fagan, Jennifer Farrar, Alicia M. Fry, Aron J. Hall, Amber Haynes, Connor Hoff, Shifaq Kamili, Marie E. Killerby, Lindsay Kim, Stephanie A. Kujawski, David T. Kuhar, Brian Lynch, Lakshmi Malapati, Mariel Marlow, Janná R. Murray, Brian Rha, Senthil Kumar K. Sakthivel, Sarah E. Smith-Jeffcoat, Elizabeth Soda, Lijuan Wang, Brett L. Whitaker, and Timothy M. Uyeki
Supplementary Material
References
- 1.WHO . World Health Organization; 2020. Coronavirus disease (COVID-19) outbreak.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [Google Scholar]
- 2.CDC . Centers for Disease Control and Prevention; 2020. Locations with confirmed COVID-19 cases, global map.https://www.cdc.gov/coronavirus/2019-ncov/locations-confirmed-cases.html#map [Google Scholar]
- 3.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phan L, Nguyen T, Luong Q, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382:872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. published online Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . World Health Organization; Jan 23, 2020. Statement on the meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)https://www.who.int/news-room/detail/23-01-2020-statement-on-the-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- 9.Park SW, Bolker BM, Champredon D, et al. Reconciling early-outbreak estimates of the basic reproductive number and its uncertainty: framework and applications to the novel coronavirus (2019-nCoV) outbreak. medRxiv. 2020 doi: 10.1101/2020.01.30.20019877. published online Feb 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC . Centers for Disease Control and Prevention; 2020. Interim U.S. guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with 2019 novel coronavirus (2019-nCoV)https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html [Google Scholar]
- 11.CDC . Centers for Disease Control and Prevention; 2020. Interim US guidance for risk assessment and public health management of persons with potential 2019 novel coronavirus (2019-nCoV) exposure in travel-associated or community settings.https://www.cdc.gov/coronavirus/2019-ncov/php/risk-assessment.html [Google Scholar]
- 12.CDC . Centers for Disease Control and Prevention; 2019. Appendix 2. Terminology. Infection control in healthcare personnel: infrastructure and routine practices for occupational infection prevention and control services (2019)https://www.cdc.gov/infectioncontrol/guidelines/healthcare-personnel/appendix/terminology.html [Google Scholar]
- 13.CDC . Centers for Disease Control and Prevention; 2020. Health alert network: update and interim guidance on outbreak of 2019 novel coronavirus (2019-nCoV)https://emergency.cdc.gov/han/han00427.asp [Google Scholar]
- 14.CDC . Centers for Disease Control and Prevention; 2020. Interim guidelines for collecting, handling, and testing clinical specimens from persons under investigation (PUIs) for 2019 novel coronavirus (2019-nCoV)https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html [Google Scholar]
- 15.CDC . Centers for Disease Control and Prevention; 2020. Research use only real-time RT-PCR protocol for identification of 2019-nCoV.https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-detection-instructions.html [Google Scholar]
- 16.CDC . Centers for Disease Control and Prevention; 2016. Infection control basics.https://www.cdc.gov/infectioncontrol/basics/index.html [Google Scholar]
- 17.Chowell G, Abdirizak F, Lee S, et al. Transmission characteristics of MERS and SARS in the healthcare setting: a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. Preliminary epidemiologic assessment of MERS-CoV outbreak in South Korea, May–June 2015. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.es2015.20.25.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Z, Ning F, Zhou W, et al. Superspreading SARS events, Beijing, 2003. Emerg Infect Dis. 2004;10:256–260. doi: 10.3201/eid1002.030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holshue M, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC . Centers for Disease Control and Prevention; 2020. Interim guidance for preventing the spread of coronavirus disease 2019 (COVID-19) in homes and residential communities.https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-prevent-spread.html [Google Scholar]
- 22.CDC . Centers for Disease Control and Prevention; 2020. Evaluating and reporting persons under investigation (PUI)https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.