FIG 5.
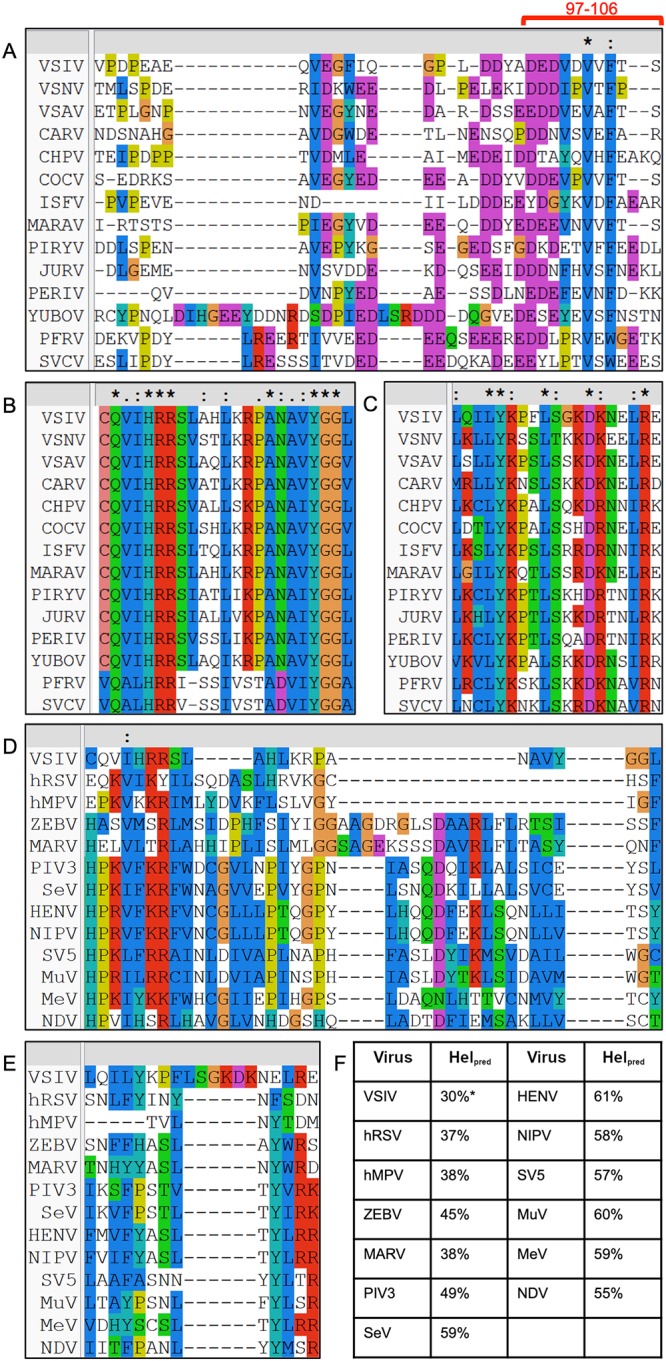
Conservation of L and P proteins. (A to C) P and L proteins from members of the Vesiculovirus and Sprivivirus genera were aligned in Clustal (37). (A) P proteins were aligned against VSV P 76 to 106. The most strongly interacting fragment, P 97 to 106, is highlighted with a red bar. (B and C) L proteins were aligned against VSV L 1414 to 1436 (B) and 1529 to 1547 (C), the two fragments implicated by HDX-MS. (D to F) L proteins from members of Mononegavirales were aligned in Clustal and subjected to secondary structure prediction. (D and E) L proteins were aligned against VSV L 1414 to 1436 (D) and 1529 to 1547 (E). (F) Secondary structure prediction was carried out for each putative connector sequence using PSIPRED (38). The predicted helical content (Helpred) is listed for each protein, except for VSV L, in which the authentic helical content is provided from the existing structure (PDB ID 5A22). VSIV, Indiana vesicular stomatitis virus; VSNV, New Jersey vesicular stomatitis virus; VSAV, Alagoas vesicular stomatitis virus; CARV, Carajas virus; CHPV, Chandipura virus; COCV, Cocal virus; ISFV, Isfahan virus; MARAV, Maraba virus; PIRYV, Piry virus; JURV, Jurona virus; PERIV, Perinet virus; YUBOV, Yug Bogdanovac virus; PFRV, pike fry virus; SVCV, spring viremia of carp virus; hRSV, human respiratory syncytial virus; hMPV, human metapneumovirus; ZEBV, Ebola virus; MARV, Marburg virus; PIV3, parainfluenza virus type 3; SeV, Sendai virus; HENV, Hendra virus; NIPV, Nipah virus; SV5, simian virus 5; MuV, mumps virus; MeV, measles virus; NDV, Newcastle disease virus. Absolute (*), strong (:), and weak (.) conservation is denoted above the alignments.
