FIG 2.
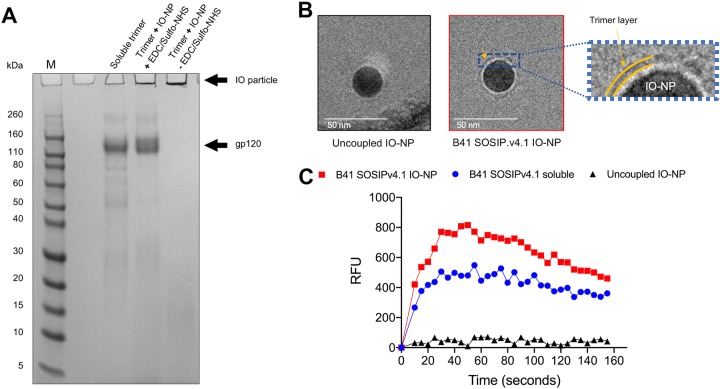
Reducing SDS-PAGE gel analysis of B41 soluble and IO-NP trimers, cryo-EM images of IO-NPs, and B-cell activation by IO-NPs. (A) SDS-PAGE gel of B41 SOSIP.v4.1 soluble and IO-NP trimers, as indicated. When sulfo-NHS and EDC were omitted, the soluble trimer was not covalently coupled to the IO-NPs (right lane). Exposure to SDS and DTT presumably dissolves the oleic acid coating on the particles and liberates the attached trimers. The positions where IO particles and gp120 migrate are shown, as are marker proteins (lane M), with the molecular masses indicated in kilodaltons. The IO-NPs do not enter the gel, but Coomassie blue-stained, released gp120 subunits migrate at the same position as those from similarly treated soluble trimers. (B) Zero-defocus cryo-EM images of uncoupled (left) and B41 SOSIP.v4.1 trimer-coupled (middle) IO-NPs. The cores of the 24-nm-diameter, monodisperse IO-NPs are clearly visible, but visualization of the surface proteins was challenging, as iron atoms not only scatter electrons but also diffract the electron beam. On the right is a zoom in of the region indicated by the yellow arrow in the middle image, to emphasize a fuzzy gray ring (yellow bands) surrounding the IO-NP core that is visible only on the trimer-bearing IO-NPs. We were unable to assess the orientation or stoichiometry of the attached trimers. (C) Ramos B cells expressing the VRC01 bNAb BCR were stimulated with 10 μg of the B41 SOSIP.v4.1 soluble (blue) or IO-NP (red) trimer or the equivalent amount of uncoupled IO-NPs (black). The recorded fluorescence outputs are representative of those from two experiments. RFU, relative fluorescence units.