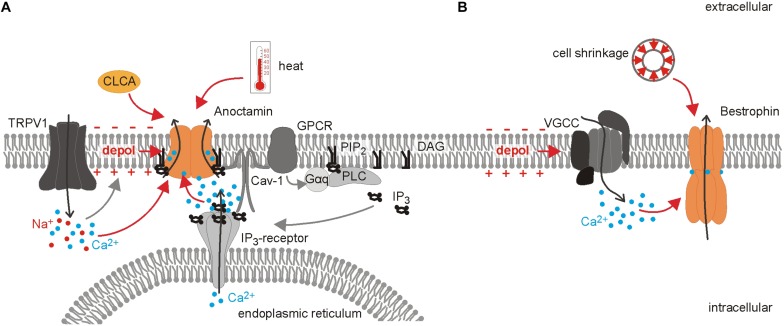
FIGURE 2.
Differential activation of Ca2+-gated Cl– channels in nociceptors. Overview of the distinct regulation of the Ca2+-gated Cl– channels anoctamin and bestrophin. (A) Ano1 is found in a signaling complex together with GPCR, Caveolin-1 (Cav-1) and the Inositol-1,4,5 trisphosphate (IP3) receptor in the membrane of the endoplasmic reticulum. This proximity of signaling molecules allows for a localized Ca2+ rise which is sufficient to activate the Cl– channel Ano1, while the Ca2+ increase mediated by VGCCs does not affect anoctamin currents. Binding of phosphatidylinositol -4,5-bisphosphate (PIP2) to anoctamin is necessary for channel opening under low Ca2+ concentration and prevents channel desensitization when Ca2+ concentration is high. Ano1 is further activated by heat and membrane depolarization. Direct interaction of TRPV1 and Ano1 leads to subsequent activation of anoctamin mediated by the TRPV1-induced depolarization and Ca2+ influx. Ano1 and TRPV1 act as peripheral heat sensors, but whether the full signaling complex exists at the peripheral nerve terminals has not been unequivocally shown so far. Additionally, the soluble N-terminus of chloride channel accessory (CLCA) increases surface availability of anoctamin. (B) In contrast to anoctamin, bestrophins can be activated by the Ca2+ influx through VGCCs and cell shrinkage.