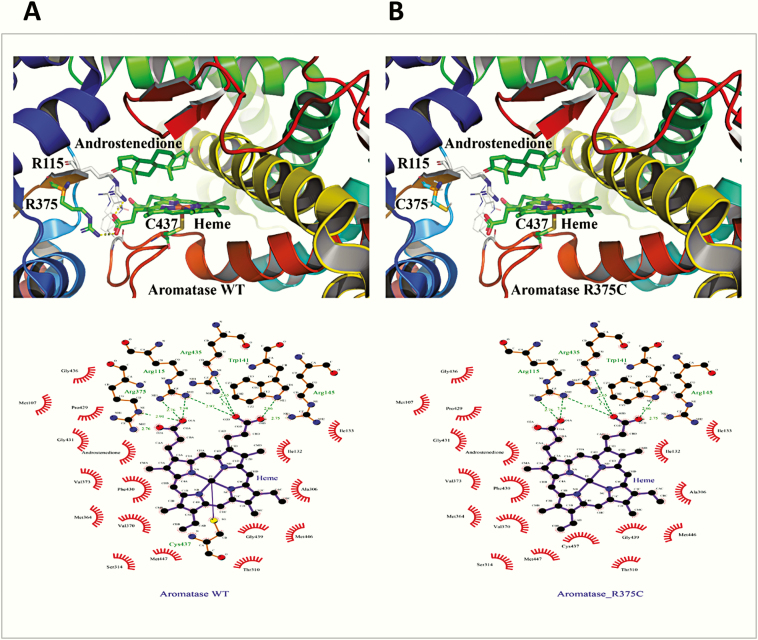
Figure 7.
Critical role of arginine 375 in heme binding. A and B, Structures of A, wild-type (WT), and B, R375C aromatase, shown as a ribbons model. The R375 residue is essential for the binding of heme at the catalytic center of the A, CYP19A1, and its change to B, cysteine, will lead to loss of heme binding, resulting in a nonfunctional protein. A complete loss of activity was observed for the R375C variant. Therefore, the arginine 375 residue has a critical role in heme binding and activity of aromatase. These observations are further substantiated by the conservation of R375 residue in aromatase across species, and no substitutions were identified in all CYP19A1 sequences analyzed (Fig. 4). C and D, A close-up of heme ligation in aromatase C, WT and D, R375C. D, After the change of arginine 375 to cysteine, one of the bonds holding the heme in place, formed between the propionate group in heme and arginine 375, is disrupted. The heme in aromatase, similar to other cytochrome P450 proteins, is held together by several bonds between the propionates of heme and arginines or histidine/tryptophan groups located at the catalytic center. Disruption of these linkages will lead to an unstable heme-binding site in aromatase and loss of heme, which would cause a nonfunctional protein. All the critical arginines (115, 145, and 375), as well as tryptophan 141 and cysteine 427, are conserved across species (Fig. 4), and no variation is observed at any of these places, confirming the importance of these amino acids in the function of aromatase.