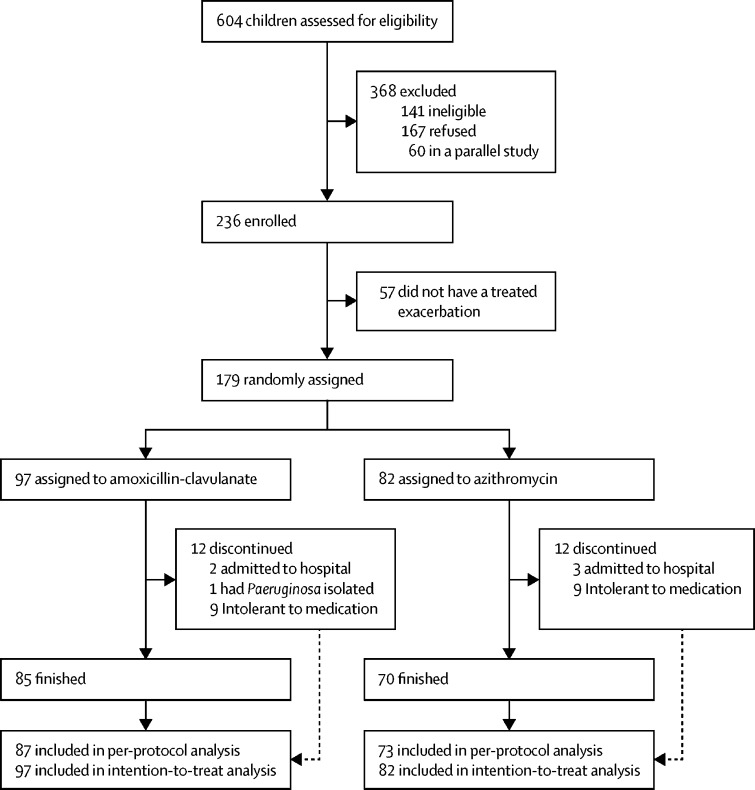
Figure 1.
Trial profile
The intention-to-treat population included all children who were randomly assigned and took at least one dose of the study medication, the per-protocol population includes children who completed treatment, including those who were admitted to hospital, but excluding one child who discontinued treatment because of Pseudomonas aeruginosa isolation and 18 who were intolerant to the treatment.