Figure 2.
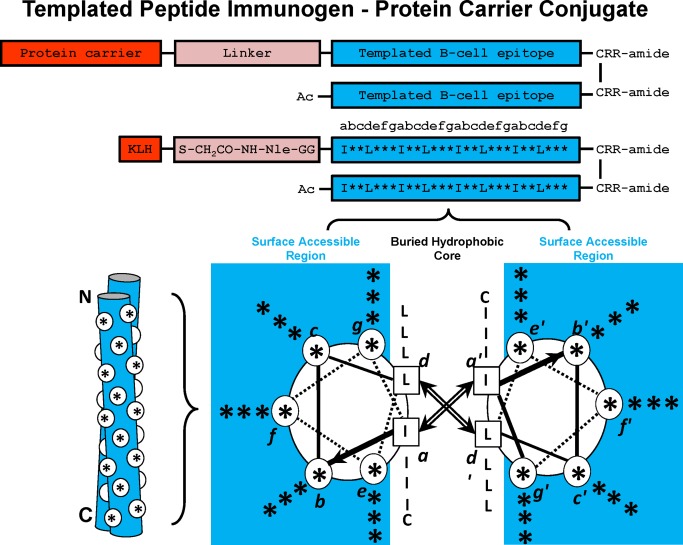
Templated α–Helical B‐cell epitopes. The top panel shows the disulfide‐bridged homo‐two‐stranded α‐helical coiled‐coil templated B‐cell epitopes. The residue positions b, c, e, f, and g, which can be substituted with the native B‐cell epitope, are indicated with an asterisk (*). The relative position of the * residues when in a two‐stranded coiled‐coil structure are shown in a cross‐sectional view (lower right) and as a cartoon (lower left). In the cross‐sectional view, the direction of the helices is into the page from the NH2 to the COOH terminus, with the polypeptide chains parallel and in‐register. Heptad positions are labeled a–g, with the prime indicating corresponding positions on the opposing helix. Arrows depict the hydrophobic interactions that occur between residues a and a′, d and d′. In the cartoon model, the white circles denote the b, c, e, f, and g positions exposed from the front view of the two‐stranded coiled‐coil. Diagram of templated peptide immunogens used in this study. Templated B‐cell epitope is conjugated to the protein carrier, Keyhole limpet hemocyanin (KLH) via a linker consisting of a GG spacer, norleucine (for quantitation of moles of peptide per mole protein carrier) and an iodoacetyl group used to couple the peptide to SH groups on KLH. The disulfide bond at the C‐terminus of the template is at an a position and is used to further stabilize the two‐stranded α–helical coiled‐coil. The RR residues at the C‐terminus are used to enhance solubility of the immunogen, if required.
