Figure 5.
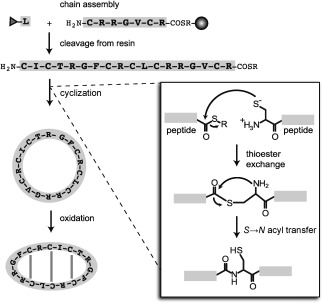
Chemical synthesis of θ‐defensins involves three stages: assembly of the peptide chain on resin; cleavage from the resin; and cyclization and oxidation to form the circular backbone and three disulfide bonds. A grey sphere represents the resin bead and amino acids are represented by one‐letter codes. The peptide chain is assembled from C to N terminus by coupling N‐terminal protected (grey triangle) amino acids to the free N terminus of the peptide chain. Inset: cyclization by native chemical ligation. The reaction involves thioester exchange between a thioester linker (COSR) at the C terminus of the peptide with the free thiol of an N‐terminal cysteine. A spontaneous S→N acyl transfer then releases the free cysteine thiol and forms a peptide bond.
