Fig. 2. The TUDOR domain of PHF20L1 is a H3K27me2-recognizing module.
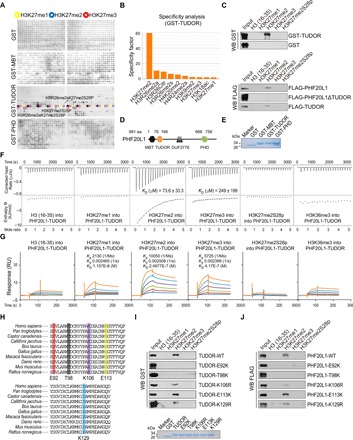
(A) Anti-GST immunoblot of the GST, GST-fused MBT, TUDOR, and PHD domains were measured on a histone peptide array. Peptides were spotted in duplicate as shown in two boxes on the same array. The positions of H3K27me1-, H3K27me2-, and H3K27me3-containing peptides are highlighted with yellow, blue, and red circles. (B) Graphical analysis of the highest binding events detected showing the binding specificity of the GST-TUDOR domain measured on a histone peptide array. (C) Western blotting analysis of histone peptide pull-down assays with GST- or FLAG-fused proteins as indicated. (D and E) Schematic illustrating the four different domains of PHF20L1 and the GST-fused domains purified from BL21 E. coli. (F) Experimental ITC titration curves of the PHF20L1 TUDOR domain to the indicated peptides. (G) SPR analysis of the interaction of PHF20L1 TUDOR with peptides as indicated. (H) Conservation of the PHF20L1 TUDOR domain among 10 species and the designated mutation amino acid sites were shown. (I and J) Western blot analysis of the peptide pull-down analysis using the GST- or FLAG-fused point mutants as indicated.
