Fig. 3. VP3 exhibits RTPase and RNA helicase activity.
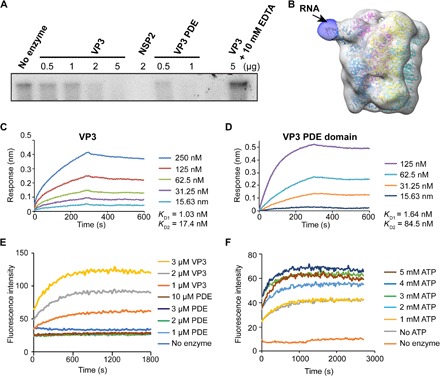
(A) An autoradiograph showing a VP3 or PDE domain concentration-dependent decrease in the 5′-end [γ-32P] GTP-labeled RNA band intensity confirms that VP3 exhibits RTPase activity and that this activity is associated with the PDE domain. RV NSP2, which was previously shown to have RTPase activity, is used as a positive control. The RTPase activity of VP3 is inhibited in the presence of EDTA indicating that the RTPase activity of VP3 is metal dependent. (B) Low-resolution cryo-EM single-particle reconstruction of VP3 in complex with 8-mer single-stranded RNA (ssRNA), suggests that the RNA binds near a cleft in the PDE domain (black arrow). The difference map was obtained using low-resolution cryo-EM map from apo-VP3 and cryo-EM map from VP3-ssRNA complex in Chimera. (C and D) Biolayer interferometry (BLI) analysis of RV VP3 and PDE domain binding with ssRNA (8-mer) shows that both full-length VP3 and PDE domain bind with ssRNA with similar nanomolar affinity. The two–binding site model fits the data with excellent R2 (~0.99) and χ2 (~0) values. (E and F) Fluorescence-based helicase assay. The 6-fluorescin amidite (6-FAM)–labeled RNA strand is quenched by a complementary quencher [Black Hole Quencher (BHQ)–labeled] strand in dsRNA. Increased fluorescence intensity with increasing concentrations of VP3 indicates the strand separation confirming the dsRNA helicase activity of VP3. Helicase activity requires the full-length VP3; the PDE domain alone does not show any significant increase in fluorescence intensity. (F) The fluorescence intensity is also modulated by adenosine triphosphate (ATP) concentrations.
