Figure 3. Integrity of the [2Fe2S] cluster is essential for the FBXL5-IRP2 interaction.
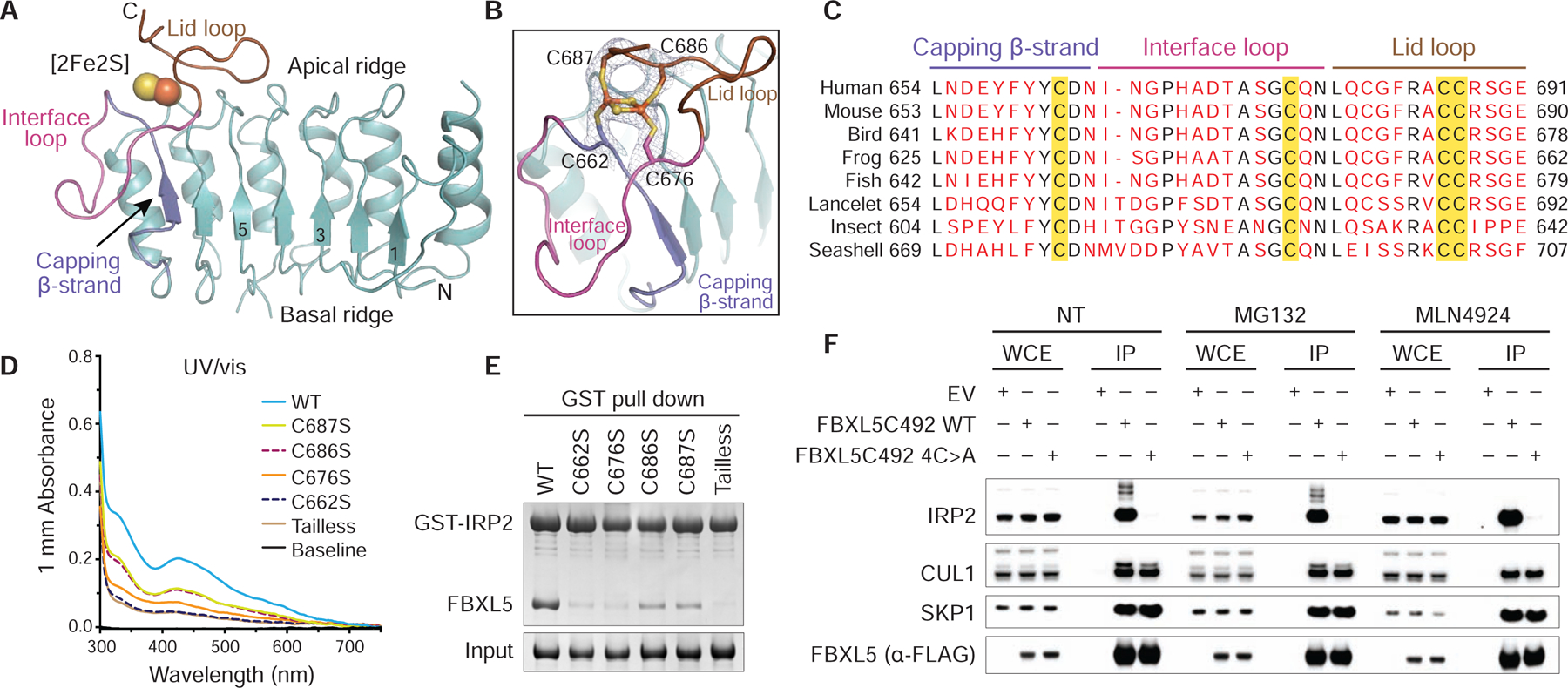
(A) Ribbon diagram of the LRR domain of FBXL5 (teal) containing the [2Fe2S] cluster (spheres). The capping β-strand (slate), interface loop (magenta) and lid loop (brown) are labeled and colored. Select LRRs are numbered and labeled.
(B) A close-up view of the [2Fe2S] cluster (spheres) ligated by four cysteines (sticks) from the capping β-strand (slate), interface loop (magenta) and lid loop (brown). A density map at 3.0 Å resolution covering the [2Fe2S] cluster and its cysteine ligands is shown in gray mesh at contour level of 2.0 σ. For clarity, the residues in the utmost C-terminus of FBXL5 are not shown.
(C) Sequence alignment of the C-terminal loop region in FBXL5 orthologs from human (Homo sapiens), mouse (Mus musculus), bird (Taeniopygia guttata), frog (Xenopus tropicalis), fish (Danio rerio), lancelet (Branchiostoma belcheri), insect (Zootermopsis nevadensis), and seashell (Oyster). Strictly conserved residues are colored black with key structural elements at the C-terminal loop region labeled. Cysteines as [2Fe2S] cluster ligands are highlighted in yellow.
(D) UV/vis absorption spectra (300–750 nm) of FBXL5 WT and mutants measured at a protein concentration of 15.0 mg/mL.
(E) Analysis of IRP2-FBXL5 interaction by GST pull-down assay using recombinant GST-IRP2 and FBXL5 WT and mutants.
(F) HEK293T cells were transiently transfected with pcDNA5/FRT/TO as an empty vector (EV) or the indicated FLAG-tagged FBXL5 plasmids. Twenty-four hours after transfection, cells were harvested and lysed. Where indicated, cells were treated with 10 μM MG132 or 2.5 μM MLN4924 for 4 hours before collection. Whole-cell extracts (WCE) were subjected to immunoprecipitation (IP) using anti-FLAG agarose beads and immunoblotting against the antibodies of FLAG, IRP2, CUL1, and SKP1.
See also Figure S5.
