SUMMARY
Background
There is an increasing body of evidence showing that earlier use of biologics improves clinical outcomes in Crohn’s disease (CD).
Aim
To perform a systematic review and meta-analysis to assess the impact of early biologic use in the treatment of CD.
Methods
PubMed and Embase databases were searched for English language papers and conference abstracts published through April 30, 2019. Studies were selected for inclusion if patients initiated biologics within 2 years of a CD diagnosis or if earlier biologics use (top-down) was compared to a conventional step-up strategy. Random-effects meta-analyses were conducted to compare clinical remission (CR), relapse and endoscopic healing rates between early biologic treatment (<2 years of disease duration or top-down treatment strategy) and late/conventional treatment (biologic use after >2 years of disease duration or conventional step-up treatment strategy).
Results
A total of 3,069 records were identified, of which 47 references met the selection criteria for systematic review. A total of 18,471 patients were studied, with a median follow-up of 64 weeks (range 10–416). Meta-analysis found that early use of biologics was associated with higher rates of clinical remission (OR 2.10 [95% CI: 1.69–2.60], n=2763, P<0.00001), lower relapse rates (OR 0.31 [95% CI: 0.14–0.68], n=596, P=0.003) and higher mucosal healing rates (OR 2.37 [95% CI: 1.78–3.16], n=994, P<0.00001) compared to late/conventional management.
Conclusions
Early biologic treatment is associated with improved clinical outcomes in both adult and paediatric CD patients, not only in prospective clinical trials but also in real-world settings.
Keywords: Crohn’s disease, meta-analyses, biologics, inflammation, outcomes research
INTRODUCTION
Crohn’s disease (CD) is a chronic, disabling, and progressive inflammatory disease of the gastrointestinal tract1–3. Chronic inflammation is associated with accumulation of tissue damage that can lead to disease complications such as strictures, fistulae and surgical resections4–6. Up to half of patients with CD will experience a disease complication requiring surgery within 10 years of diagnosis7.
The treatment paradigm has shifted to a treat-to-target approach in CD in which endoscopic healing is paramount in order to improve remission rates and long-term risk of complications8. Despite their higher efficacy, biologics are often used as a later line of therapy for Crohn’s disease (CD), after steroids, 5-ASA or thiopurines9,10. There is an increasing volume of evidence suggesting that early use of biologics can improve rates of remission and disease complications11–17. Despite these data, the rates of early adoption of biologic therapy are quite low in real world practice18. Therefore, we conducted a systematic review and meta-analysis of the current available data on the impact of early biologic use in CD.
MATERIALS AND METHODS
Study Identification
A systematic review of English language abstracts in the following databases was performed per a pre-specified and clearly defined PRISMA guidelines-based protocol through April 30, 2019: Medline, Medline In-Process and Embase. Search terms comprised combinations of free-text and medical subject heading (MeSH) terms. The criteria for screening of the articles were as follows:
Population: All patients with Crohn’s disease (e.g. “Crohn’s Disease” [MeSH])
Interventions of interest: Adalimumab, Infliximab, Certolizumab pegol, Natalizumab, Vedolizumab, Ustekinumab, or any combinations of these treatments
Early treatment related terms: “early treatment”, “earlier treatment”, “disease duration”, “late treatment”, “top down”
Study type of interest: All types of studies except case reports and case series
Relevant conference proceedings, Internet resources and backward snowballing (bibliographic reference lists of any identified systematic reviews and meta-analyses were searched)
Study Selection
Studies were excluded if they did not include patients with disease duration less than 2 years. All titles and abstracts were reviewed by two co-authors to determine if they met the study screening criteria (Level 1 screen); those selected in the first screen underwent a full text review for relevance (Level 2 screen). For quality control, a third individual manually checked the screening criteria based on titles and abstracts for all included and excluded studies. For each eligible study, the relevant data were extracted in duplicate and independently verified with the original sources. Outcomes included clinical remission (CR), corticosteroid free clinical remission (CSFR), deep remission, endoscopic healing, relapse rate, hospitalization rate, complications (defined as progression to strictures and/or fistulas/abscess), IBD-related surgeries, cost effectiveness and safety in adult and paediatric populations.
Meta-Analysis
A random-effects meta-analysis (Cochrane RevMan 5.3) was conducted to compare efficacy of early with late or conventional treatment with biologics. Early biologic treatment was defined as treatment initiated within 2 years of disease diagnosis or “top-down” therapy where biologics were initiated prior to oral immunosuppressants. Late/conventional treatment was defined as treatment initiated after 2 years of disease duration or conventional step-up management (i.e., use after oral immunosuppressants). Data from clinical trials and observational studies were pooled and analysed in meta-analysis if similar criteria were used to define study populations, outcomes and measurement timing. The late/conventional group data was pooled if there were data for two or more disease durations (e.g. 2–5, 5–10 and >10 years). For efficacy, pooled odds ratios (OR) for CR, relapse rates and mucosal healing (MH) rates were assessed using the Mantel Haenszel (M-H) method. The analysis was conducted for overall, adult and paediatric patient groups, respectively, as appropriate. Sensitivity analyses were conducted by excluding observational studies and use of biologics in combination with immunomodulators (IM) or immunosuppressants (IS). Inter-study heterogeneity was assessed with Cochrane Q and I2 tests (with significant heterogeneity indicated by P<0.10 or I2 ≥50%). A quality assessment for observational studies and post hoc analyses was conducted for studies included in the meta-analysis using the New-Castle Ottawa Scale19. For RCTs, risk of bias assessment was conducted using the tool developed by UK’s National Institute for Health and Care Excellence (NICE; https://www.nice.org.uk/process/pmg24/chapter/clinical-effectiveness).
RESULTS
Search Results
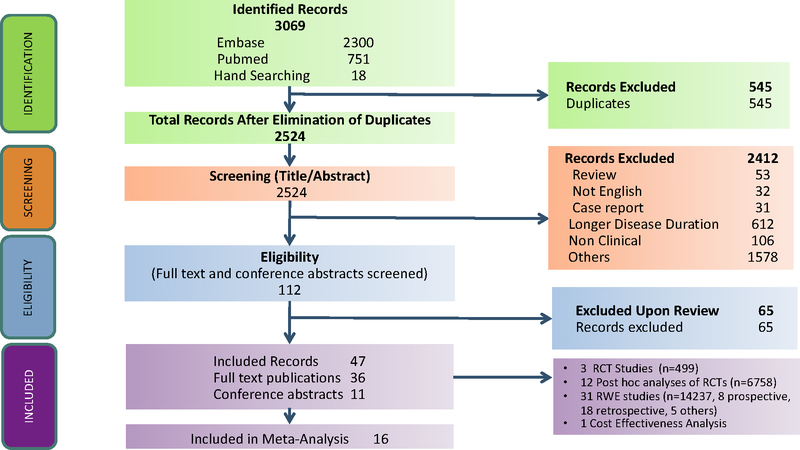
A total of 3069 records were identified and screened. Forty-seven studies were included after applying inclusion and exclusion criteria including three randomized controlled trials (RCTs) (n=499 patients, Table 1A), 12 post hoc analyses of RCTs (n=6758 patients, Table 1A), 31 observational studies (n=14237 patients, Table 1B) and one cost-effectiveness analysis model met the inclusion criteria (Figure 1). Among the 31 observational studies, eight were prospective studies (n=3905 patients), 18 were retrospective studies (n=5797 patients), and five studies (n=4535 patients) lacked details of study design. A total of 18,471 patients were studied, with a median follow-up duration of 64 weeks (range 10–416). Twenty-eight studies were conducted in adult patients, 16 studies were conducted in paediatric patients and three studies were conducted in adult and paediatric patients. In most studies, use of biologics within 1 year or 2 years of disease diagnosis was defined as “early” biologic use. Seven studies were conducted in newly diagnosed patients. Of the 47 studies that met inclusion/exclusion criteria, sixteen studies met the criteria for meta-analysis (based on comparative design, outcomes definitions, follow up period). The range of quality assessment score for observational studies and post analyses included in the meta-analysed study was 5–8 (out of maximum score of 8), indicating a high quality of the included studies (Supplementary Table S6). The two RCTs included in the meta-analysis also were of relatively high quality (Supplementary Table S7).
Table 1A.
Study Characteristics (RCT and Post-hoc Analysis of RCTs)
| Author Journal/Conference Year | Study type | Patient | Size | Study Duration | Intervention(s) | Disease Duration Definitions for Evaluating Early Biologic Use |
|---|---|---|---|---|---|---|
| Panaccione, J Crohns Colitis 201921 | Post hoc analysis | Adult | 2207 | NR | ADA | < 2 yrs, 2 to 5 yrs, >5 yrs |
| Dulai, ECCO, 201939 | Post hoc analysis | Adult | 1253 | 7 yrs | VDZ | ≤2 vs. >2, ≤3 vs. >3, and ≤5 vs. >5 yrs |
| Reinisch, ECCO, 200920 | Post hoc analysis | Adult† | 945 | 20 wks | ADA | <2 yrs, 2 to 5 yrs, >5 yrs |
| Schreiber, J Crohns Colitis., 201311 | Post hoc analysis (RCT and open label extension) | Adult | 777 | 3 yrs | ADA | <2 yrs, 2 to <5 yrs, ≥5 yrs |
| Rubin, DDW, 200932 | Post hoc analysis | Adult | 491 | 48 wks | ADA, PBO | 2 yrs, ≥2 yrs |
| Schreiber, Am J Gastroenterol., 201012 | Post hoc analysis | Adult | 425 | 26 wks | CER | <1 year, ≥1 to < 2 yrs, ≥2 to <5 yrs, ≥5 yrs |
| Colombel, Lancet, 201829 | RCT | Adult | 244 | 48 wks | ADA | ≤2 yrs, >2 yrs |
| Colombel, Aliment Pharmacol Ther., 201517 | Post hoc analysis | Adult | 188 | 26 wks | AZA, IFX and AZA+IFX | ≤18 mon, >18 mon |
| D’Haens, Lancet, 200815 | RCT | Adult | 129 | 2 yrs | IFX+AZA vs AZA | Newly diagnosed |
| Feagan, Gastroenterology, 201443 | RCT | Adult | 126 | 50 wks | IFX+MTX, IFX+PBO | <2 yrs |
| Yzet, ECCO, 201924 | Post hoc analysis | Adult | 122 | 3.02 yrs | ADA | ≤2 yrs, >2 yrs |
| Sandborn, DDW, 201027 | Post hoc analysis | Adult | 123 | 1 year | ADA, PBO | <2 yrs, 2 to 5 yrs, >5 yrs |
| Hyams, ECCO, 200951 | Post hoc analysis | Paediatric | 112 | 54 wks | IFX+IM | <2 yrs, ≥2 yrs |
| Colombel, Clin Gastroenterol Hepatol., 201416 | Post hoc analysis | Adult | 69 | 1 year | ADA | <2 yrs, 2 to 5 yrs, >5 yrs |
| Baert, Gastroenterology., 201030 | Extension study (follow up of an RCT) | Adult | 46 | 2 yrs | IFX+AZA vs AZA | Newly diagnosed |
ADA: adalimumab, IFX: infliximab; PBO: placebo; AZA: azathioprine; CER: certolizumab; IM: immunomodulators; IS: immunosuppressants NR: Not reported, VDZ: Vedolizumab; yrs: years.
Assumed to be adult.
Table 1B.
Study Characteristics (Observational Studies)
| Author Journal/Conference Year | Design | Patient | Size | Study Duration | Intervention(s) | Disease Duration Definitions for Evaluating Early Biologic Use | |
|---|---|---|---|---|---|---|---|
| Rubin, Inflamm Bowel Dis., 201236 | Observational | Adult | 3750 | 2 yrs | ‘Step-Up’, IS-to-TNF’, ‘Early-TNF’ | ≤30 days | |
| Loftus, Inflamm Bowel Dis., 201945 | Observational study | Adult | 1980 | 1 year | ADA | <2 yrs, 2–<5 yrs 5–<10 yrs, ≥10 yrs | |
| Ogata, J Crohns Colitis., 201622 | Observational | Adult | 1693 | 24 wks | ADA | <2, 2 to 5, 5–10, 10–20, >20 yrs | |
| † | Kerur, CGH 201855 | Observational | Paediatric | 1442 | 10 yrs | Anti-TNFs | <3 months |
| Kugathasan, Lancet., 201749 | Observational | Paediatric | 913 | 3 yrs | IFX, ADA, IM | Newly diagnosed | |
| Faleck, CGH, 201923 | Observational | Adult | 650 | 6 months | VDZ | ≤2 yrs, >2–≤5 yr, >5 yrs | |
| Walters, Gastroenterology., 201447 | Observational | Paediatric | 552 | 1 year | Anti-TNF or IM (not specified) | Newly diagnosed | |
| Safroneeva, Aliment Pharmacol Ther., 201533 | Observational | Adult | 540 | 15 mon | IM and/or TNF antagonists | <2 yrs | |
| Oh, PLoS One., 201737 | Observational | Adult | 507 | NR | Anti-TNF or IM | <2 yrs | |
| Safroneeva, ECCO, 201334 | Observational | Adult | 450 | 15 mon | IM or Anti-TNF | <2 yrs (adjusted for diagnostic delay) | |
| Seitz, UEG 201831 | Retrospective chart review | Adult | 242 | 2 years | Anti-TNFs | <2 and >2 years | |
| Church, Inflamm Bowel Dis., 201457 | Observational | Paediatric | 195 | 8 yrs | IFX | Median 1.6 (0.6–3.0) yrs | |
| Ma, Inflamm Bowel Dis., 201638 | Observational | Adult | 190 | 154 wks§ | IFX, ADA | <2 yrs | |
| Kotze, Digestion., 201542 | Observational | Adult | 175 | NR | ADA, IFX | <2 yrs | |
| Chambrun, ECCO, 201540 | Observational | Adult‡ | 153 | 44 mon (mean) | IFX | < 18 mon | |
| Yu, Mediators Inflamm., 201526 | Observational | Adult and Paediatric | 106 | 10 wks | IFX | 22.2 mon (remission group) | |
| Wauters, Inflamm Bowel Dis., 201761 | Observational | Paediatric | 91 | 5 yrs | Anti-TNF (not specified) | 0.2 (0.1–0.5) yrs | |
| Panchal, DDS 201944 | Retrospective chart review | Adult and Paediatric | 88 | NR | Anti-TNFs w/o IM | < 2, 3–10, 11–20, 21–30 and >30 yrs | |
| Nuij, J Crohns Colitis., 201541 | Observational | Adult and Paediatric | 85 | 41.4 mon§ | IFX, IFX+ADA, ADA | Newly diagnosed, <16 mon | |
| † | Bolia, J Pediatr Gastroenterol Nutr., 201756 | Observational | Paediatric | 73 | 1 year | IFX w/o IM in early versus non-early CD | Time interval between diagnosis and introduction of IFX (mon): Loss of response group: 28 (4–90); No loss of response group: 12.5 (1–121) |
| Lee, J Pediatr Gastroenterol Nutr., 201553 | Observational | Paediatric | 51 | 3 yrs | AZA+IFX (top-down) versus step-up | 10.8 ± 9.0 (step-up) mon vs 1.0 ± 0.5 (top-down) mon | |
| † | Kato, Gut Liver., 201146 | Observational | Adult | 43 | 14 wks | IFX | 3.26±5.63 mon |
| Ling, DDS, 201860 | Retrospective chart review | Paediatric | 43 | 48 months | IFX | <3 months and ≥ 3 months | |
| † | Nuti, J Crohns Colitis., 201548 | Observational | Paediatric | 37 | 2 yrs | IFX, ADA | 13 ± 16 (0.5–63) mon |
| † | Olbjørn, Scand J Gastroenterol., 201428 | Observational | Paediatric | 36 | 2 yrs | IFX | Newly diagnosed |
| Lee, World J Gastroenterol., 201054 | Observational | Paediatric | 36 | 2 yrs | AZA+IFX, AZA | Newly diagnosed | |
| Kim, Acta Paediatr., 201150 | Observational | Paediatric | 29 | 1 year | AZA+IFX (top-down) vs step-up | Step-up: 11.5 ± 7.4; Top-down: 0.8 ± 0.6 | |
| Lee, Pediatr Gastroenterol Hepatol Nutr., 201252 | Observational | Paediatric | 28 | 3 yrs | AZA+IFX (top-down) versus step-up | Duration from the initial diagnosis to IFX infusion: Step-up: 49.6±5.2; Top-down: 1.8±2.4 wks | |
| † | Wewer, J Pediatr Gastroenterol Nutr., 200659 | Observational | Paediatric | 24 | 3 yrs | IFX (episodic) | 26 (range 0.7–93) mon |
| Lionetti, Aliment Pharmacol Ther., 200358 | Observational | Paediatric | 22 | 18 wks | IFX | <1 year | |
| Echarri, DDW 201135 | Observational | Adult | 13 | 6 mon | ADA, IFX | <2 yrs | |
| Beilman, CGH, 201862 | Model | Adult | N/A | Lifetime | ADA, IFX | <2 yrs |
ADA: adalimumab, IFX: infliximab; PBO: placebo; AZA: azathioprine; CER: certolizumab; IM: immunomodulators; IS: immunosuppressants NR: Not reported, VDZ: vedolizumab.
Studies marked
were not comparative studies
Assumed to be adult
Median follow up.
Note: Nuij 2015 had 66 Crohn’s disease [CD], 16 ulcerative colitis [UC], 3 inflammatory bowel disease unclassified [IBDU]) patients, NR is not reported
Figure 1.
PRISMA Diagram of Study Screening and Selection
Systematic Review Results: Efficacy Outcomes
Efficacy in Adults
Eight studies reported higher clinical remission rates (Table 2) with early treatment (38%−81.5%) versus late/conventional treatment (14%−80%)11,12,15,17,20–23. In an RCT, the CR rates for early versus late/conventional were 60.6% vs. 35.9% (p=0.0062)15. In a large pooled post hoc analysis of ten trials, the CR rates were significantly higher in the shorter disease duration groups (<1 year: 45.8%, ≥1–<2 years: 31.0%; 2–≤5 years: 23.1%; >5 years: 23.6%, p = 0.026)21. In two other post hoc analyses, the CR rates in early versus late treatment groups were 56% vs 58% (n=945, <2 vs 2–5 years at 20 weeks, CR was defined as HBI≤4)20 and 46% vs 28% (n=777, <2 vs 2–≤5 years at 26 weeks)11. Two large observational studies reported similar trend in higher remission rates in early versus late/conventional treatment group22,23.
Table 2.
Clinical Remission in Adults
| Study (year) | N | Outcome | Timepoint | Results |
|---|---|---|---|---|
| Panaccione 201921 | 2207 | CR (CDAI<150) | 26 weeks | <1 year: 57.8% (n=45) ≥1–<2 years: 35.8% (n=67) 2–≤5 years: 34.2% (n=196) >5 years: 33.7% (n=768) p=0.013 |
| Ogata 2016†22 | 1693 | CR (CDAI < 150) | 24 weeks | <2 versus 2 to <5 years: OR 0.84 (0.26–2.65) p=0.76 <2 versus 5 to <10 years: OR 0.28 (0.10–0.80) p=0.02 <2 versus 10 to <20 years: OR 0.27 (0.10–0.75) p=0.01 <2 versus >20 years: OR 0.24 (0.08–0.71) p=0.01 |
| Schreiber 201012 | 425 | CR (CDAI ≤ 150) | 26 weeks | <1 Year: 68.4% (n=19) ≥1 to <2 years: 55.0% (n=20) ≥2 to <5 years: 46.7% (n=45) ≥5 Years: 44.3% (n=131) |
| Faleck 201923 | 650 | CR (complete resolution of all CD related symptoms) And CSFR (tapering off steroids completely, achieving clinical remission, and no repeat steroid prescription within 4 weeks of tapering) | 26 weeks | CR ≤ 2 years vs >2 years: 38% vs 23% (p<0.05) CSFR ≤ 2 years vs >2 years: 43% vs 14% (p<0.05) |
| Schreiber 201311 | 777 | CR (CDAI < 150) | 26 weeks | <2 years: 46% (n=56) ≥2 to <5 years: 28% (n=95) ≥5 years: 32% (n=366) |
| 52 weeks | <2 years: 43% (n=56) ≥2 to <5 years: 30% (n=95) ≥5 years: 28% (n=366) |
|||
| Reinisch 200920 | 945 | CR (HBI ≤ 4) | 20 weeks | <2 years: 58% (n=62/107) 2–5 years 56% (n=121/217) >5 years 50% (n=309/621) |
| Colombel 201517 | 188 | CR (CDAI < 150) and Composite Outcomes | 26 weeks | IFX+AZA Combination early versus non-early: CR: 81.5% vs 80.0% p<0.05 CR+MH: 63% vs 53.3% p<0.05 CR+CRPnorm: 76.5% vs 55.6% p>0.05 CR+MH+CRPnorm: 64.7% vs 44.4% p<0.05 |
| D’Haens 200815 | 129 | CSFR (CDAI < 150 and absence of corticosteroid treatment, and no intestinal resection) | 26 weeks | Early combined immunosuppression group vs conventional management: •60.6% (n=39/65) vs 35.9% (n=23/64) •Δ=24.1% (95% CI 7.3–40.8, p=0.0062) |
| 52 weeks | Early combined immunosuppression group vs conventional management: •61.5% (n=40/65) vs 42.2% (n=27/64) •Δ=19.4% (95% CI 2.4–36.3, p=0.0278) |
Study only reported odds ratios.
Two studies reported deep remission rates of 33–36.9% with early biologic treatment compared to 10–23% with late/conventional management16,24,25. In a 3-year follow up study, patients with early CD who had deep remission were less likely to have disease progression24.
Nine publications showed that early treatment leads to better mucosal healing rates (29.0–72.0%) compared to late/conventional treatment (12.9–62.2%)15,17,23,26–31. In an RCT the rate of mucosal healing was 45.9% vs 30.3% (n=244, p=0.010) in tight control vs conventional treatment group29. In a long term follow-up of an RCT, significantly higher MH rates of 72.0% compared to 28.6% were observed in early versus late/conventional treatment group30. Similar trends were observed in five observational studies in a real-world setting23,26,28,31.
In a post-hoc analysis of hospital admissions (measured week 4 through week 56), hospitalization-free rates were higher although not significant, in patients with shorter disease duration (<2 yr 93%; 2–5 yr 90%; >5 yr 86%)11. One study reported significantly lower relapse rates in early versus late/conventional treatment group32. Three studies observed that compared to late/conventional treatment, early treatment with biologics reduced bowel strictures and damage33–35. One RCT, six observational studies and one post-hoc analysis showed significantly lower rates of intestinal surgery in early versus late/conventional treatment group15,34,36–40. However, one study showed that early treatment did not reduce the rate of IBD complications in early versus late/conventional treatment group41. Four additional studies showed potential value of early treatment with lower loss of efficacy42, decreased time to treatment failure43, slower rate of disease progression44 and improvement in mean HBI scores45. A small study showed 91% CR rate in an early treatment group46. See Supplementary Table S1 for efficacy in adults.
Efficacy in Paediatric Patients
Five studies in paediatric patients reported significantly higher CR rates in early treatment groups (62%−85%) versus late/conventional treatment (45.5–60.3%) (Table 3)47–51. Three studies reported lower relapse rates in early (16.1%−23.1%) versus late/conventional treatment groups (50.0%−61.5%)52–54. Two small studies reported mucosal healing rates of 45% vs 32%48 and 65% vs 62%28 when comparing early versus late/conventional treatment group. Two studies reported surgery outcomes, one showed lower rates at 1 year with early biologics compared to early immunosuppressants (2.94% vs 1.61%)53 and the other study showed that 10-year risk for first bowel surgery was 26%55. Two studies provide evidence for lowering risk of penetrating complications with early biologic treatment49,55, though one study showed that early treatment might not lower risk of stricturing complications49. Five studies showed value of early treatment for loss of response56, better height57, higher PCDAI score58, prolonged response rate59 and sustained primary response60. In one observational study outcomes were similar with accelerated step-up and conventional management61. See Supplementary Table S2 for efficacy in paediatric patients.
Table 3.
Clinical Remission in Paediatric Patients
| Study (year) | N | Outcome | Timepoint | Results |
|---|---|---|---|---|
| Kim 201150 | 29 | CR (PCDAI<10) | 1 year | Step-up: 5 of 11 Top-down: 15 of 18 |
| Hyams 200951 | 52 | CR (PCDAI≤10) | 54 weeks | <2 years: 62% (n=26) ≥2 years: 50% (n=26) |
| Walters 201447 | 136 | CR (Corticosteroid free and PCDAI≤10) | 1 year | Early anti-TNF: 85% (n=68) Early IM: 60% (n=68) |
| Nuti 201548 | 37 | CR (absence of symptoms related to CD and PCDAI ≤10) | 2 year | Early (<1 year) group: 70.6% Late (>1 year) group: 70% |
| Kugathasan 201749 | 175 | Corticosteroid free remission rate | 26 weeks | 71% (124/175) |
Cost Effectiveness Outcomes
One study was found that assessed cost effectiveness of early (within 2 years of CD diagnosis) versus late (more than 2 years after diagnosis) treatment with biologics62. In a Canadian setting, a Markov model was developed to simulate the progression of a hypothetical cohort of patients with CD after the initiation of either infliximab or adalimumab. The model compared the lifetime cost-effectiveness of early versus late initiation of anti-TNF therapy using published loss of response rates. The model results show that over a patient’s lifetime, early initiation of infliximab yielded an additional 1.02 quality-adjusted life years (QALYs) and saved $18,054 compared to late initiation of infliximab. Similarly, early initiation of adalimumab yielded an additional 0.74 QALYs and saved $18,526 compared to late initiation of adalimumab. At a willingness-to-pay threshold of $50,000 per QALY, early initiation of both infliximab and adalimumab had a 68% chance of being cost-effective, while late initiation had a 32% chance of being cost-effective (Supplementary Table S3).
Safety
In a large pooled analysis (n=2207), the overall rate of adverse events (AEs), serious adverse events (SAEs), AEs leading to discontinuation and malignancies were similar between disease duration groups21. Serious infection rates were lower in those treated early versus patients treated within 2–5 yrs and >5 yrs of CD diagnosis (4.2 vs 8.7 and 8.3/100 PY)21. In an open label 2-year randomized control trial, the rate of serious adverse events were similar in early combined immunosuppression and conventional management groups (30.8% versus 25.3%, p=1.0)15. In a post hoc analysis of an RCT, the incidence of serious adverse events was lowest with disease duration less than 2 years (14% in <2 years group, versus 22.7% and 22.0% in 2–5 years and ≥5 years group, respectively)11. In a post hoc analysis of a randomized maintenance trial there were more serious adverse events with increasing disease duration (SAEs < 1 year: 0%, ≥1 to <2 years: 0%, ≥ 2 to <5 years: 6.7% and ≥ 5 Years: 6.8%)12. Overall across studies, the serious infection or malignancy rates were similar or better in early compared to late biologic groups. See Supplementary Table S4 for safety outcomes.
Meta-Analysis: Efficacy Outcomes
Clinical Remission
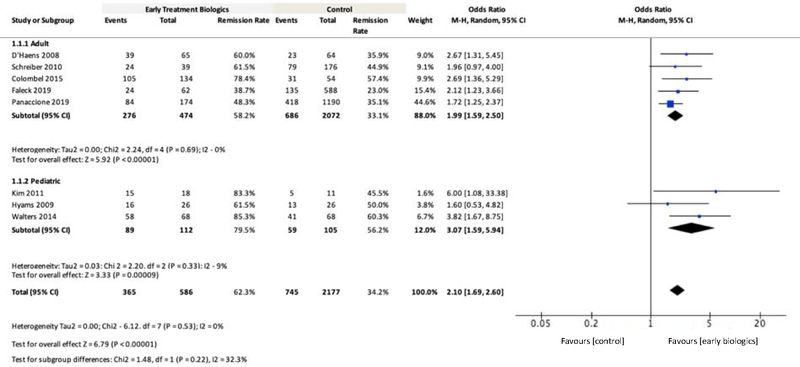
Eight publications reporting clinical remission in adult12,15,17,21,23 and paediatric47,50,51 patients were included for meta-analysis. Overall higher rates of CR at 26–52 week ranged from 48–78% in early biologic treatment compared to 35–57% for late or conventional management or step-up (Figure 2). The overall pooled remission rates in adult patients at 26 week were 62.3% (95% CI: 58.2%−66.2%) and 34.2% (95% CI: 32.2%−36.3%) in early biologic treatment versus late/conventional, respectively (n=2763). For the overall patient population, the OR for remission with early biologic treatment was 2.10 (95% CI: 1.69–2.60, n=2763, P<0.00001, Figure 2) compared to the late/conventional treatment. The results were similar in adult and paediatric patient subgroups. In adults, the OR for remission at 26 week with early biologic treatment was 1.99 (95% CI: 1.59–2.50, n=2546, P<0.00001, Figure 2). In the paediatric patient subgroup, the OR for remission at ~52 week with early biologic treatment was 3.07 (95% CI: 1.59–5.94, n=217, P=0.0009, Figure 2). The OR were similar for studies using only biologics (no IM/IS; Supplementary Figure 2) and using only RCTs (Supplementary Figure 3). Of the publications that included clinical remission, three reported CSFR in adult and paediatric patients15,23,47. The OR for CSFR with early biologic treatment was 3.83 (95% CI: 2.60–5.63, n=720, P<0.00001, Supplementary Figure 1).
Figure 2.
Meta-Analysis Comparing Clinical Remission Rates for Early Biologic versus Late/Conventional Treatment
Notes: Definition of clinical remission: three studies used CDAI<15012, 17,21; one study used CDAI<150 plus no bowel resection and no steroid use15; two studies used PCDAI≤1050,51; one study used corticosteroid free remission and PCDAI ≤10 at 1 year after diagnosis without luminal resection47; one study did not provide definition of clinical remission23
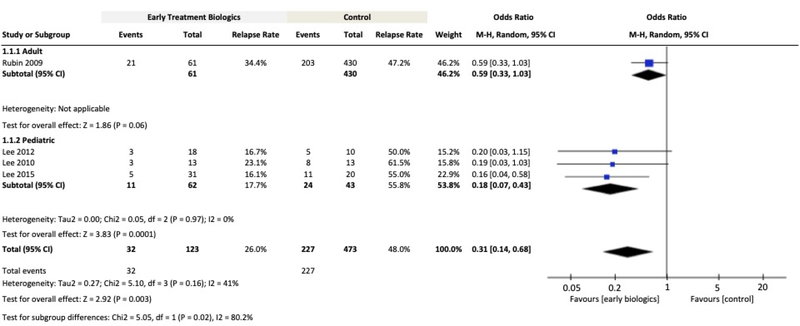
Relapse Rate
Four publications reporting relapse rates in adult32 and paediatric 52–54 patients were included for meta-analysis. For the overall patient population, the OR for relapse with early biologic treatment was 0.31 (95% CI: 0.14–0.68, n=596, P=0.003, Figure 3) compared to late/conventional treatment. In the paediatric patient subgroup, the OR for relapse at 1 year with early biologic treatment was 0.18 (95% CI: 0.07–0.43, n=105, P=0.0001 Figure 3). Subgroup analysis for adults was not feasible because only one study reported relapse rates comparing early versus late treatment.
Figure 3.
Meta-Analysis Comparing Relapse Rates for Early Biologic versus Late/Conventional Treatment
Definition of disease relapse: One study used increase in CDAI≥70 and an absolute CDAI>22032; three studies used PCDAI>1052–54
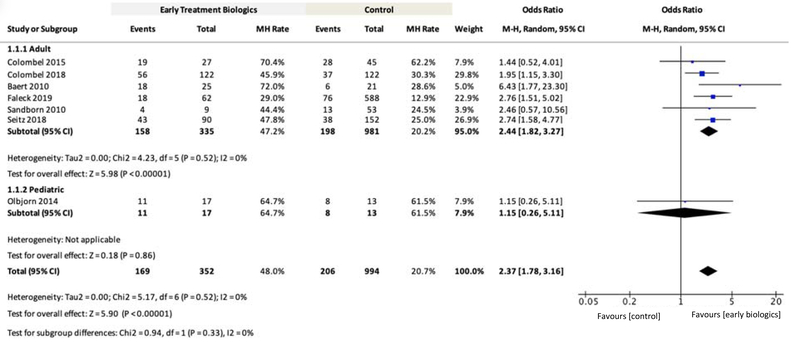
Mucosal Healing Rate
Seven publications reporting mucosal healing (MH) rates at 12 week to 4 years in adult15,17,23,27,29,31 and paediatric28 patients were included for meta-analysis. For the overall patient population, the OR for MH rate with early biologic treatment was 2.37 (95% CI: 1.78–3.16, n=994, P<0.00001, Figure 4) compared to late/conventional treatment. In the adult patient subgroup, the OR for MH rate with early biologic treatment was 2.44 (95% CI: 1.82–3.27, n=981, P<0.00001, Figure 4). Subgroup analysis for paediatrics was not feasible because only one study reported MH rates comparing early versus late treatment. The results for overall OR were similar for only RCTs (Supplementary Figure 4).
Figure 4.
Meta-Analysis Comparing Mucosal Healing (MH) Rates for Early Biologic versus Late/Conventional Treatment
Definition of endoscopic healing: one study used SES-CD=015; one study used CDEIS<4 and absence of deep ulcers29; one study used absence of any mucosal ulcers (including aphthous ulcers)17; one study used absence of mucosal ulceration27; one study used absence of ulcers and/or erosions23; one study used disappearance of ulcerations, multiple erosions, bleeding and friability (grade 0 or 1)28; one study did not report definition31
DISCUSSION
To our knowledge, this is the most comprehensive systematic review and meta-analysis on outcomes of early biologic use in CD. The meta-analysis findings confirm our hypothesis that early use of biologics in patients with moderate-severe CD leads to statistically and clinically better clinical remission (OR 2.10 [95% CI: 1.69–2.60], n=2763, P<0.00001), relapse (OR 0.31 [95% CI: 0.14–0.68], n=596, P=0.003) and mucosal healing (2.37 (95% CI: 1.78–3.16, n=994, P<0.00001) rates compared to late/conventional treatment. We observed that early biologic treatment is associated with improved clinical outcomes (clinical remission, corticosteroid free remission, mucosal healing, relapse rate, hospitalization rate, complications and surgeries) not only in prospective clinical trials but also in real-world settings. Lower rates of hospitalizations and surgery suggest there may be decreased resource use with early use of biologics with one study suggesting that early biologic use is cost-effective.
Furthermore, multiple sensitivity analyses using data only from RCTs, studies with CSFR and by excluding studies that used biologics in combination with IM/IS confirmed our hypothesis of value of early treatment with biologics in CD. Using data only from RCTs, the OR for clinical remission with early use of biologics was 1.95 (n=564, 95% CI:1.52–2.49, P<0.00001) versus late/conventional treatment. The OR was similar for mucosal healing rates in RCTs (OR 2.21 [95% CI: 1.34–3.64], n=241, P=0,002). Using studies without any combination treatment with IM/IS, the OR for clinical remission was 2.09 (n=2033, 95% CI:1.56–2.80, P<0.00001). The OR for pooled CSFR rate for early biologic use versus late/conventional treatment was 3.83 (n=146, 95% CI:2.60–5.63, P<0.00001).
A review comparing trials in early CD and rheumatoid arthritis (RA) suggests that the concept of early intervention in other immune-mediated diseases such as RA is well established and there might be lessons to learn from other chronic inflammatory diseases63. This systematic review and meta-analysis supports findings and hypotheses from several other narrative reviews and overviews3,63–69.
Despite this evidence, the real-world utilization of biologics is still low in early disease CD patients18. There is a need to educate clinicians, policymakers and payer stakeholders to improve access and utilization of biologics in early disease settings, especially for patients with moderate to severe CD. Though our study supports early use of biologics, there are some limitations of our review. First, the outcome definitions slightly differed across some studies. Second, the follow up duration varied across trials. Third, the vast majority of data were on anti-TNF agents alone so our results may not be generalizable to other therapies. There are still limited data on longer-term outcomes such as prevention of complications, bowel damage, disability, and durability on drug. Further studies should continue to understand if early intervention with a more treat to target / tight control approach impacts long term outcomes, and if the improved response rates with shorter disease duration applies to all classes of biologics/targeted agents.
Supplementary Material
ACKNOWLEDGEMENT
Declaration of personal interests
Jean-Frederic Colombel has served as a consultant or advisory board member for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, Celltrion, Enterome, Ferring, Genentech, Janssen and Janssen, Medimmune, Merck & Co., Pfizer, Protagonist, Second Genome, Seres, Shire, Takeda, Theradiag; a speaker for AbbVie, Ferring; speaker’s bureau for Amgen. Dr. Colombel has received research grants from Takeda, Johnson and Johnson, and is a stockholder of Intestinal Biotech Development and Genefit.
Ryan Ungaro is supported by a Career Development Award from the Crohn’s and Colitis Foundation and an NIH K23 Career Development Award (K23KD111995-01A1). He has served as an advisory board member or consultant for Janssen, Pfizer, and Takeda and received research grants from AbbVie, Boehringer Ingelheim, and Pfizer.
Saurabh Aggarwal and Ozlem Topaloglu are employees of NOVEL Health Strategies, which received payment from AbbVie to assist with the literature review.
Ryan Clark and Wan-Ju Lee are employees of AbbVie and may hold AbbVie stock.
Declaration of funding interests
Financial support for the study was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication. All authors contributed to the development of the publication and maintained control over the final content.
Medical writing support was provided by Sushil Kumar, of NOVEL Health Strategies, Columbia, MD, USA; this support was funded by AbbVie.
References
- 1.Danese S, Fiorino G, Fernandes C, Peyrin-Biroulet L. Catching the therapeutic window of opportunity in early Crohn’s disease. Current drug targets. 2014;15(11):1056–1063. [DOI] [PubMed] [Google Scholar]
- 2.Torres J, Mehandru S, Colombel J-F, Peyrin-Biroulet L. Crohn’s disease. Lancet (London, England). 2017;389(10080):1741–1755. [DOI] [PubMed] [Google Scholar]
- 3.Berg DR, Colombel J-F, Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflammatory bowel diseases. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflammatory bowel diseases. 2002;8(4):244–250. [DOI] [PubMed] [Google Scholar]
- 5.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn’s disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49(6):777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV, Jr. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139(4):1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peyrin-Biroulet L Why should we define and target early Crohn’s disease? Gastroenterol Hepatol. 2011;7(5):324–326. [PMC free article] [PubMed] [Google Scholar]
- 8.Serban ED. Treat-to-target in Crohn’s disease: Will transmural healing become a therapeutic endpoint? World J Clin Cases. 2018;6(12):501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. The American journal of gastroenterology. 2018;113(4):481–517. [DOI] [PubMed] [Google Scholar]
- 10.Ruemmele FM, Veres G, Kolho KL, et al. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. Journal of Crohn’s & colitis. 2014;8(10):1179–1207. [DOI] [PubMed] [Google Scholar]
- 11.Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. Journal of Crohn’s & colitis. 2013;7(3):213–221. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. The American journal of gastroenterology. 2010;105(7):1574–1582. [DOI] [PubMed] [Google Scholar]
- 13.Sandborn WJ. Initial combination therapy in early Crohn’s disease. Lancet (London, England). 2008;371(9613):635–636. [DOI] [PubMed] [Google Scholar]
- 14.Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet (London, England). 2015;386(10006):1825–1834. [DOI] [PubMed] [Google Scholar]
- 15.D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet (London, England). 2008;371(9613):660–667. [DOI] [PubMed] [Google Scholar]
- 16.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2014;12(3):414–422 e415. [DOI] [PubMed] [Google Scholar]
- 17.Colombel JF, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naive patients with Crohn’s disease - a SONIC post hoc analysis. Alimentary pharmacology & therapeutics. 2015;41(8):734–746. [DOI] [PubMed] [Google Scholar]
- 18.Siegel CA, Yang F, Eslava S, Cai J. Real-world treatment pathway visualizations show low use of biologic therapies in Crohn’s disease and ulcerative colitis in the United States. Gastroenterology. 2017;152(5):S371–S372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. http://wwwohrica/programs/clinical_epidemiology/oxfordasp 2001.
- 20.Reinisch W, Lofberg R, Louis E, et al. P125-Influence of disease duration on adalimumab efficacy in Crohn’s disease: subanalysis of the CARE trial. Journal of Crohn’s & colitis. 2009;3(1):S60. [Google Scholar]
- 21.Panaccione R, Rutgeerts P, Sandborn WJ, et al. Adalimumab efficacy and safety by disease duration: analysis of pooled studies of Crohn’s disease. Journal of Crohn’s & colitis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata H, Watanabe M, Matsui T, et al. Safety of adalimumab and predictors of adverse events in 1693 Japanese patients with Crohn’s disease. Journal of Crohn’s & colitis. 2016;10(9):1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faleck DM, Winters A, Chablaney S, et al. Shorter disease duration is associated with higher rates of response to vedolizumab in patients with Crohn’s disease but not ulcerative colitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yzet C, Ungaro R, Bossuyt P, et al. OP35 Endoscopic and deep remission at 1 year prevents disease progression in early Crohn’s disease: long-term data from CALM. Journal of Crohn’s & colitis. 2019;13(Supplement_1):S024–S025. [Google Scholar]
- 25.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet (London, England). 2018;390(10114):2779–2789. [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Yang X, Xia L, et al. Infliximab preferentially induces clinical remission and mucosal healing in short course Crohn’s disease with luminal lesions through balancing abnormal immune response in gut mucosa. Mediators of inflammation. 2015;2015:793764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandborn WJ, Panaccione R, Thakkar R, et al. S1031 Crohn’s disease mucosal healing in adalimumab-treated patients is affected by disease duration: results from EXTEND. Gastroenterology. 2010;138(5):S–164. [Google Scholar]
- 28.Olbjorn C, Nakstad B, Smastuen MC, Thiis-Evensen E, Vatn MH, Perminow G. Early anti-TNF treatment in pediatric Crohn’s disease. Predictors of clinical outcome in a population-based cohort of newly diagnosed patients. Scandinavian journal of gastroenterology. 2014;49(12):1425–1431. [DOI] [PubMed] [Google Scholar]
- 29.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet (London, England). 2018;390(10114):2779–2789. [DOI] [PubMed] [Google Scholar]
- 30.Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138(2):463–468; quiz e410–461. [DOI] [PubMed] [Google Scholar]
- 31.Seitz T, Schnitzler F, Borchardt J, Howaldt S, Janelidze S. Early versus late intervention with anti-TNFα- antibodies in Crohn’s disease: effect on mucosal healing, development of strictures and need for resective surgery. A retrospective cohort analysis. United European Gastroenterol J. 2018;6(8):A108. [Google Scholar]
- 32.Rubin DT, Bensimon AG, Andrew PY, Wu E, Chao J, Mulani P. S1136 Increased risk of clinical relapse is associated with longer disease duration in Crohn’s disease. Gastroenterology. 2009;136(5):A-197. [Google Scholar]
- 33.Safroneeva E, Vavricka SR, Fournier N, et al. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Alimentary pharmacology & therapeutics. 2015;42(8):977–989. [DOI] [PubMed] [Google Scholar]
- 34.Safroneeva E, Vavricka S, Fournier N, Rogler G, Straumann A, Schoepfer A. P393 Occurrence of stricturing and penetrating complications is diminished in Crohn’s disease patients treated by immunomodulatory and/or anti-TNF therapy within the first two years of disease duration when corrected for diagnostic delay. Journal of Crohn’s & colitis. 2013;7:S167. [Google Scholar]
- 35.Echarri A, Gallego JC, Ollero V, Porta A, Castro JA. Can early anti-TNF treatment modify the outcome of inflammatory strictures? Gastroenterology. 2011;140(5):S–779. [Google Scholar]
- 36.Rubin DT, Uluscu O, Sederman R. Response to biologic therapy in Crohn’s disease is improved with early treatment: an analysis of health claims data. Inflammatory bowel diseases. 2012;18(12):2225–2231. [DOI] [PubMed] [Google Scholar]
- 37.Oh EH, Oh K, Han M, et al. Early anti-TNF/immunomodulator therapy is associated with better long-term clinical outcomes in Asian patients with Crohn’s disease with poor prognostic factors. PloS one. 2017;12(5):e0177479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma C, Beilman CL, Huang VW, et al. Anti-TNF therapy within 2 years of Crohn’s disease diagnosis improves patient outcomes: a retrospective cohort study. Inflammatory bowel diseases. 2016;22(4):870–879. [DOI] [PubMed] [Google Scholar]
- 39.Dulai P, Peyrin-Biroulet L, Hahn K, et al. P537 The impact of early disease control with vedolizumab on surgery rates among patients with Crohn’s disease: a post-hoc analysis of the GEMINI trials. Journal of Crohn’s & colitis. 2019;13(Supplement_1):S382–S383. [Google Scholar]
- 40.Chambrun GP, Libier L, Nachury M, et al. Early treatment with infliximab for Crohn’s disease patients. Journal of Crohn’s & colitis. 2015;9:S251–S252. [Google Scholar]
- 41.Nuij V, Fuhler GM, Edel AJ, et al. Benefit of earlier anti-TNF treatment on IBD disease complications? Journal of Crohn’s & colitis. 2015;9(11):997–1003. [DOI] [PubMed] [Google Scholar]
- 42.Kotze PG, Ludvig JC, Teixeira FV, et al. Disease duration did not influence the rates of loss of efficacy of the anti-TNF therapy in Latin American Crohn’s disease patients. Digestion. 2015;91(2):158–163. [DOI] [PubMed] [Google Scholar]
- 43.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology. 2014;146(3):681–688 e681. [DOI] [PubMed] [Google Scholar]
- 44.Panchal H, Wagner M, Chatterji M, et al. Earlier anti-tumor necrosis factor therapy of Crohn’s disease correlates with slower progression of bowel damage. Dig Dis Sci. 2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loftus EV, Reinisch W, Panaccione R, et al. Adalimumab effectiveness up to six years in adalimumab-naïve patients with Crohn’s disease: results of the PYRAMID registry. Inflammatory bowel diseases. 2019;25(9):1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kato K, Fukunaga K, Kamikozuru K, et al. Infliximab therapy impacts the peripheral immune system of immunomodulator and corticosteroid naive patients with Crohn’s disease. Gut and liver. 2011;5(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walters TD, Kim MO, Denson LA, et al. Increased effectiveness of early therapy with anti-tumor necrosis factor-alpha vs an immunomodulator in children with Crohn’s disease. Gastroenterology. 2014;146(2):383–391. [DOI] [PubMed] [Google Scholar]
- 48.Nuti F, Civitelli F, Bloise S, et al. Prospective evaluation of the achievement of mucosal healing with anti-TNF-alpha therapy in a paediatric Crohn’s disease cohort. Journal of Crohn’s & colitis. 2016;10(1):5–12. [DOI] [PubMed] [Google Scholar]
- 49.Kugathasan S, Denson LA, Walters TD, et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet (London, England). 2017;389(10080):1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MJ, Lee JS, Lee JH, Kim JY, Choe YH. Infliximab therapy in children with Crohn’s disease: a one-year evaluation of efficacy comparing ‘top-down’ and ‘step-up’ strategies. Acta paediatrica (Oslo, Norway : 1992). 2011;100(3):451–455. [DOI] [PubMed] [Google Scholar]
- 51.Hyams J, Crandall W, Kugathasan S, et al. P192-Disease duration does not affect outcome following infliximab therapy in children with Crohn’s disease. Journal of Crohn’s & colitis. 2009;3(1):S86. [Google Scholar]
- 52.Lee YS, Baek SH, Kim MJ, Lee YM, Lee Y, Choe YH. Efficacy of early infliximab treatment for pediatric Crohn’s disease: a three-year follow-up. Pediatric gastroenterology, hepatology & nutrition. 2012;15(4):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee YM, Kang B, Lee Y, Kim MJ, Choe YH. Infliximab “top-down” strategy is superior to “step-up” in maintaining long-term remission in the treatment of pediatric Crohn disease. Journal of pediatric gastroenterology and nutrition. 2015;60(6):737–743. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Lee JH, Lee JH, et al. Efficacy of early treatment with infliximab in pediatric Crohn’s disease. World journal of gastroenterology. 2010;16(14):1776–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerur B, Machan JT, Shapiro JM, et al. Biologics delay progression of Crohn’s disease, but not early surgery, in children. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;16(9):1467–1473. [DOI] [PubMed] [Google Scholar]
- 56.Bolia R, Rosenbaum J, Schildkraut V, et al. Secondary loss of response to infliximab in pediatric Crohn disease: does it matter how and when we start? Journal of pediatric gastroenterology and nutrition. 2018;66(4):637–640. [DOI] [PubMed] [Google Scholar]
- 57.Church PC, Guan J, Walters TD, et al. Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn’s disease. Inflammatory bowel diseases. 2014;20(7):1177–1186. [DOI] [PubMed] [Google Scholar]
- 58.Lionetti P, Bronzini F, Salvestrini C, et al. Response to infliximab is related to disease duration in paediatric Crohn’s disease. Alimentary pharmacology & therapeutics. 2003;18(4):425–431. [DOI] [PubMed] [Google Scholar]
- 59.Wewer V, Riis L, Vind I, Husby S, Munkholm P, Paerregaard A. Infliximab dependency in a national cohort of children with Crohn’s disease. Journal of pediatric gastroenterology and nutrition. 2006;42(1):40–45. [DOI] [PubMed] [Google Scholar]
- 60.Ling J, Buurman D, Ravikumara M, Mews C, Thacker K, Grover Z. Accelerated step-up infliximab use is associated with sustained primary response in pediatric Crohn’s disease. Dig Dis Sci. 2018;63(4):1003–1010. [DOI] [PubMed] [Google Scholar]
- 61.Wauters L, Smets F, De Greef E, et al. Long-term outcomes with anti-TNF therapy and accelerated step-up in the prospective pediatric Belgian Crohn’s Disease Registry (BELCRO). Inflammatory bowel diseases. 2017;23(9):1584–1591. [DOI] [PubMed] [Google Scholar]
- 62.Beilman CL, Kirwin E, Ma C, McCabe C, Fedorak RN, Halloran BP. Early initiation of tumor cecrosis factor antagonist–based therapy for patients with Crohn’s disease reduces costs compared with late initiation. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2018;17(8):1515–1524. [DOI] [PubMed] [Google Scholar]
- 63.Danese S, Fiorino G, Peyrin-Biroulet L. Early intervention in Crohn’s disease: towards disease modification trials. Gut. 2017;66(12):2179–2187. [DOI] [PubMed] [Google Scholar]
- 64.Walters TD, Hyams JS. Can early anti-TNF-α treatment be an effective therapeutic strategy in children with Crohn’s disease? Immunotherapy. 2014;6(7):799–802. [DOI] [PubMed] [Google Scholar]
- 65.Spurio FF, Aratari A, Margagnoni G, Doddato MT, Papi C. Early treatment in Crohn’s disease: do we have enough evidence to reverse the therapeutic pyramid? J Gastrointestin Liver Dis. 2012;21(1). [PubMed] [Google Scholar]
- 66.D’Haens GR. Top-down therapy for IBD: rationale and requisite evidence. Nat Rev Gastroenterol Hepatol. 2010;7(2):86–92. [DOI] [PubMed] [Google Scholar]
- 67.Hirschmann S, Neurath MF. Top-down approach to biological therapy of Crohn’s disease. Expert Opin Biol Ther. 2017;17(3):285–293. [DOI] [PubMed] [Google Scholar]
- 68.Peyrin-Biroulet L Why should we define and target early Crohn’s disease? Gastroenterology & hepatology. 2011;7(5):324. [PMC free article] [PubMed] [Google Scholar]
- 69.Kang B, Choe YH. Early biologic treatment in pediatric Crohn’s disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatric gastroenterology, hepatology & nutrition. 2018;21(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.