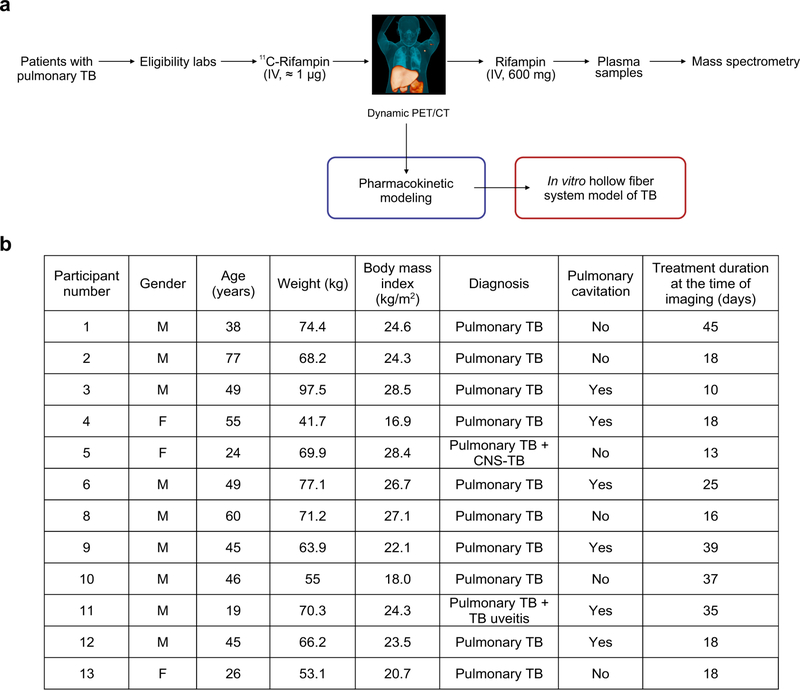
Extended Data Fig. 1 |. Study outline and patient characteristics.
(a) Patients with pulmonary TB receiving a rifampin-based TB treatment were enrolled and 11C-rifampin PET/CT performed within 6-weeks of treatment initiation. A subset of patients (n = 2) was also imaged at least 20 weeks after starting treatment. (b) All patients were HIV negative and were receiving an oral regimen of isoniazid, rifampin, pyrazinamide, and ethambutol at the time of imaging, except for patient 5 that received moxifloxacin instead of ethambutol. Patient 7 was excluded from the study due to significant motion artifact during the 11C-rifampin PET/CT. Gender: male (M) and female (F).