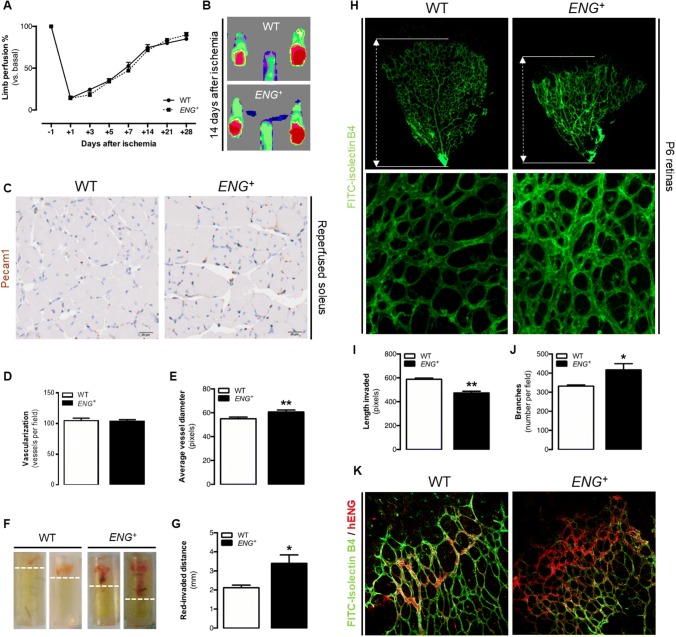
Fig. 2.
Continuous endoglin overexpression impairs in vivo angiogenesis. a Ratio of ischemic to non-ischemic limb perfusion following femoral artery ligation as measured by laser Doppler flow analysis in mice, represented as the percentage of the basal value (before artery ligation) at 1, 3, 5, 7, 14, 21 and 28 days post-ischemia [n(WT) = 9, n(ENG+) = 9; p = 0.4989]. b Laser Doppler images showing mice hindlimb perfusion 14 days after ischemia. c Pecam1 immunostaining in the ischemic soleus muscle 14 days post-ischemia. d Quantification of the number of Pecam1-positive vessels in the ischemic soleus muscle [n(WT) = 4, n(ENG+) = 6; p = 0.8204]. e Average Pecam1-positive vessel diameter in the ischemic soleus muscle [n(WT) = 3, n(ENG+) = 3; p = 0.0098]. f DIVAA 9 days after implantation in mice, showing blood invasion. g Quantification of DIVAA red-invaded distance from the tube end 9 days after implantation [n(WT) = 16, n(ENG+) = 28; p = 0.0383]. h Upper panel: FITC-lectin staining of ECs in the retinal vasculature of p6 pups and representation of the plexus progression. Lower panel: Structure of the vessel plexus of the retina in P6 pups. i Quantification of plexus progression in the retinas of P6 pups [n(WT) = 7, n(ENG+) = 3; p < 0.0001]. j Quantification of the ramification of the retinas of P6 pups [n(WT) = 7, n(ENG+) = 3; p = 0.0227]. k Human endoglin (red) and FITC-lectin (green) staining in the angiogenic front of the retinal vasculature of P6 pups. The angiogenic front is oriented towards the upper left of the image in both cases