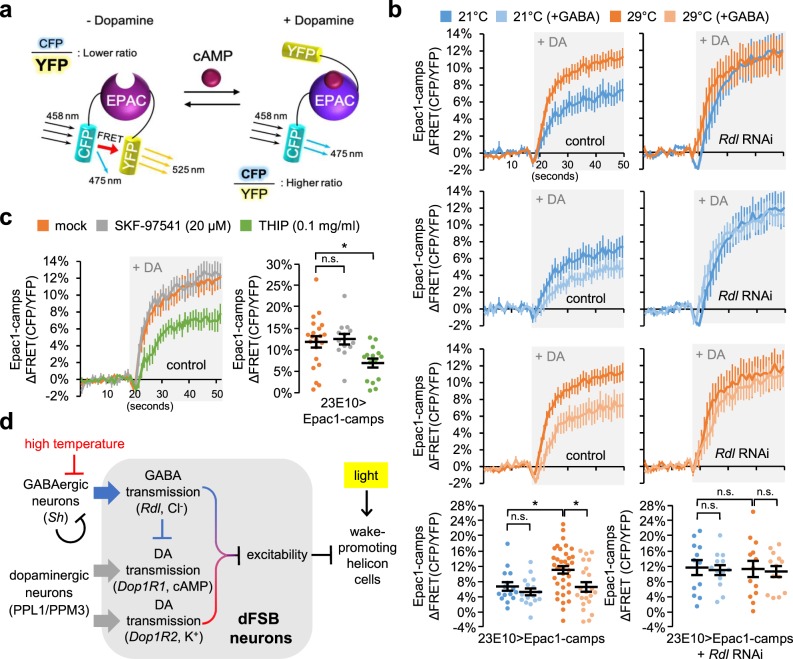
Fig. 7. Ionotropic GABA transmission suppresses postsynaptic DA signaling in dFSB neurons and supports temperature-sensitive DA transmission.
a DA receptor signaling elevates intracellular cyclic AMP (cAMP) levels. The subsequent binding of cAMP to Epac1-camps (EPAC) induces conformational change in the FRET sensor, thereby increasing the fluorescence ratio of CFP to YFP. b Epac1-camps was expressed in dFSB neurons of control (23E10 > Epac1-camps) or Rdl RNAi flies (23E10 > Epac1-camps + Rdl RNAi). Transgenic flies were pre-entrained in LD cycles at 21 °C (blue) or 29 °C (orange). Whole brains were dissected out and transferred to an imaging chamber. Where indicated, dissected brains were pre-incubated with 1 mM GABA for 5 min prior to the induction of FRET responses by the batch application of 10 mM DA (shaded by gray boxes). A time series of the fluorescence images was recorded using a multi-photon microscopy and their FRET analysis was performed using ZEN software. Data represent average ± SEM (n = 15–37 for 23E10 > Epac1-camps; n = 12–13 for 23E10 > Epac1-camps + Rdl RNAi). Two-way ANOVA detected significant effects of either temperature or GABA on the DA-induced FRET response in control (P = 0.0238 or P = 0.0151, respectively), but not in Rdl-depleted dFSB neurons (P = 0.7894 or P = 0.7040, respectively). n.s., not significant; *P < 0.05 as determined by Tukey post hoc test. c 23E10 > Epac1-camps flies were pre-entrained in LD cycles at 29 °C. Where indicated, dissected brains were pre-incubated with SKF-97541 (20 µM, a metabotropic GABA receptor agonist) or THIP (0.1 mg/ml, an ionotropic GABA receptor agonist) for 5 min prior to the induction of FRET responses by the batch application of 10 mM DA. Data represent averag ± SEM (n = 13–21). n.s., not significant; *P < 0.05 as determined by one-way ANOVA, Tukey post hoc test. d A model for the genetic and neural interplay of sleep-promoting dFSB neurons that supports temperature- and light-sensitive plasticity of sleep behaviors.