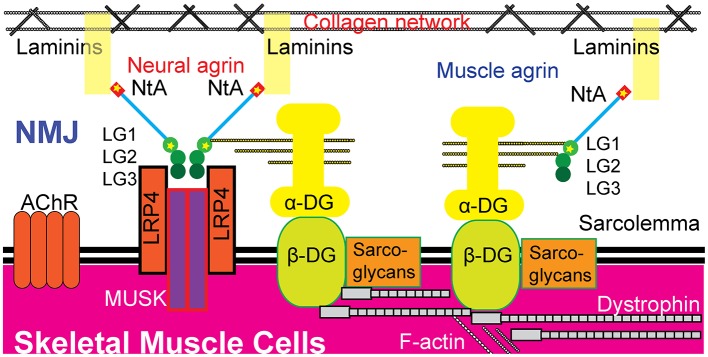
Figure 3.
Possible pathogenic mechanisms of the two novel heterozygous missense mutations in AGRN. The p.Glu42 and p.Ala1506 residues exist in both neural agrin and muscle agrin. The p.Glu42Ala mutation was located in the NtA domain, which binds to laminin in the BL. The p.Ala1506Thr mutation was located in the LG1 domain, which binds to α-dystroglycan (α-DG). Neural agrin forms an agrin-LRP4 binary complex during AChR aggregation and LG1 has no direct role during this process. Whether or not p.Ala1506Thr interferes with AChR aggregation is unknown. Additionally, muscle agrin may act as a collateral linker by binding to the coiled-coil of laminins and to α-dystroglycan to function critically in the maintenance of the NMJ. Hence, these two mutations are hypothesized to potentially disrupt the formation and maintenance of the NMJ through both neural and muscle agrin pathways, leading to CMS. AChR, acetylcholine receptor; DG, dystroglycan; LG, laminin G-like domain; LRP4, low-density lipoprotein receptor-related protein 4; MUSK, muscle-specific kinase; NtA, N-terminal agrin domain; NMJ, neuromuscular junction.