Table 3.
The published studies of PF regimen concurrent with radiotherapy in postoperative recurrent ESCC
| Reference | Trial type | n | Groups | Chemotherapy regimen | Radiation dose, Gy | ORR, % | mPFS | mOS | Survival rate | Hematological toxicities | Nonhematological toxicities |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jingu et al., 2006 2 | Phase II | 30 | RT+PF (31) | Nedaplatin: 70 mg/m2/2 h; 5‐FU: 500 mg/m2/24 h D1–5; 3 wk; 2 cycles | 60 | 73.70 | NA | 39 | 1‐y OS: 60.6%; 3‐y OS: 56.3% 1‐y PFS: 53.4%; 3‐y PFS:35.6% | Grade 3–4: 10 (33.3%) | Grade 3–4: 3 (10%) |
| Lu et al., 2010 14 | Retrospective | 73 | RT (42) | NA | 60 | 76 | NA | 9 | 1‐y OS: 33.8%; 3‐y OS: 0% | 0 | 0 |
| RT+PF (31) | 5‐FU: 750 mg/m2 on D1–5; cisplatin: 20 mg/m2 on D1–5 or 40 mg/m2 on D1–3; ≥2 cycles | 97 | 17 (p = .049) | 1‐y OS: 62.5%; 3‐y OS: 10.5% | Grade 3–4: 9 (29%) | Grade 3–4: 16 (52%) | |||||
| Bao et al., 2013 1 | Retrospective | 83 | RT+PF (39) |
1. Cisplatin: 60 mg/m2 on D1, 29; 5‐FU: 300 mg/m2 on D1–3 and 29–31; 2 cycles; 2. Cisplatin: 30 mg/m2 with 5‐FU: 500 mg/m2 on D1–5 and 29–33; 2 cycles; |
60 (56–68) | 75.9 | NA | 43 | 3‐y OS: 43.3% | Grade 3–4: 6 (23.1%) | Grade 3 vomiting: 8 (30.8%); grade 3 esophagitis: 2 (5.1%) |
| RT+TP (44) |
1. Docetaxel: 60 mg/m2, and cisplatin: 80 mg/m2 on D1,29: 2 cycles; 2. Docetaxel: 30 mg/m2 on D1; cisplatin: 30 mg/m2 on D1; weekly for 4–6 wk (n = 15) |
3‐y OS: 59.2% | Grade 3–4: 11 (36.7%) | Grade 3 vomiting: 10 (33.3%); grade 3 esophatitis: 3 (6.8%) | |||||||
| Zhang et al., 2012 19 | Retrospective | 50 | RT+PF (22) | 5‐FU: 500 mg/m2 on D1–D5; cisplatin: 75 mg/m2 on D1; 4 wk; 1–2 cycles | 60 (50.4–64) | 72.7 | 9.8 | 9.8 | NA | Grade 3–4: 7 (31.8%) | Grade 3–4: 6 (27.3%) |
| RT+TP (28) | Paclitaxel: 135 mg/m2 on D1; cisplatin: 75 mg/m2 on D1; 3 wk; 1–2 cycles | 71.7 | 9.8 | 16.3 (p = .012) | NA | Grade 3–4: 6 (21.4%) | Grade 3–4: 8 (28.6%) | ||||
| Zhang et al., 2015 4 | Retrospective | 27 | RT+CF (13) | Cisplatin: 25 mg/m2 on D1, 5‐FU: 300 mg/d on D 1–3, weekly for 4 wk | 56 (46.0–60.0) | 70.4 | 16.4 | 26 | 1‐y OS: 88.9%; 2‐y OS: 60.2%; 3‐y OS: 43.3% | Grade 3: 3 (21.4%) | Grade 3: 3 (7.1%) |
| RT+NF (14) | Nedaplatin: 25 mg/m2 on D1, 5‐FU: 300 mg/d on D1–3, weekly for 4 wk |
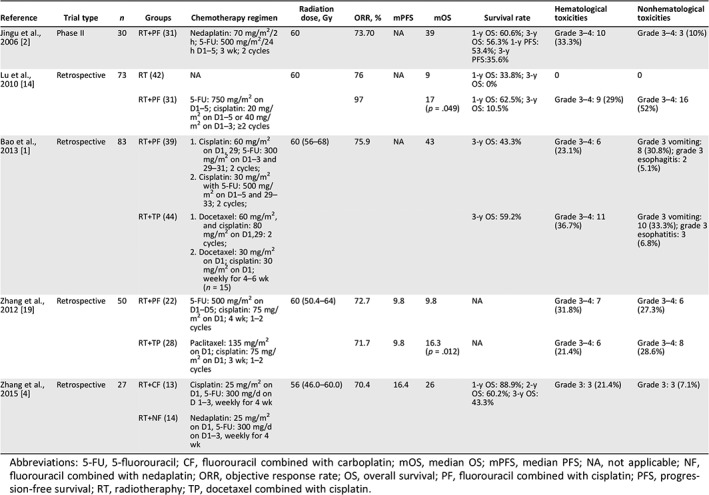
Abbreviations: 5‐FU, 5‐fluorouracil; CF, fluorouracil combined with carboplatin; mOS, median OS; mPFS, median PFS; NA, not applicable; NF, fluorouracil combined with nedaplatin; ORR, objective response rate; OS, overall survival; PF, fluorouracil combined with cisplatin; PFS, progression‐free survival; RT, radiotheraphy; TP, docetaxel combined with cisplatin.
