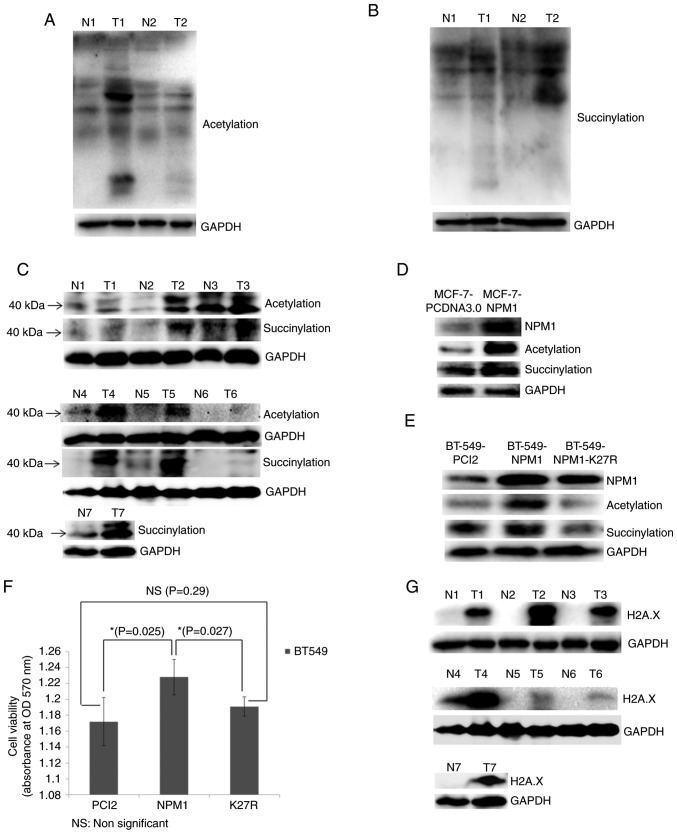
Figure 5.
Validation of proteomic results. (A) Acetylation and (B) succinylation of whole proteins in breast cancer tissues (T) and adjacent normal tissues (N) examined via western blotting. GAPDH was used as a loading control. (C) Acetylation and succinylation of proteins ~40 kDa (used for indicating NPM1) in breast cancer tissues (T) and adjacent normal tissues (N) examined via western blotting. GAPDH was used as a loading control. (D) MCF-7 cells overexpressing NPM1 (MCF-7-NPM1) and PCDNA3.0 (MCF-7-PCDNA3.0, used as control) were collected for the protein extraction. Expression level of NPM1 and its acetylation and succinylation were detected via western blotting. GAPDH was used as a loading control. (E) BT549 cells overexpressing NPM1 (BT549-NPM1), PCI2 (BT549-PCI2, used as control) and the variants of NPM1 (NPM1-K27R, lysine mutated into arginine) were collected for protein extraction. Expression level of NPM1 and its acetylation and succinylation were detected via western blotting. GAPDH was used as a loading control. (F) Viability of BT549 cells overexpressing NPM1 (NPM1), PCI2 (PCI2, used as control) and the variants of NPM1 (K27R, lysine mutated into arginine) was detected via MTT methods. *P<0.05. (G) Expression of H2A.X in breast cancer tissues (T) and adjacent normal tissues (N) were detected via western blotting. GAPDH was used as a loading control. The H2A.X and the succinylation bands from N4 to T6 shows non-adjacent bands from the same gel, therefore they used the same GAPDH result. NPM1, nucleophosmin; H2A.X, histone H2A.X.