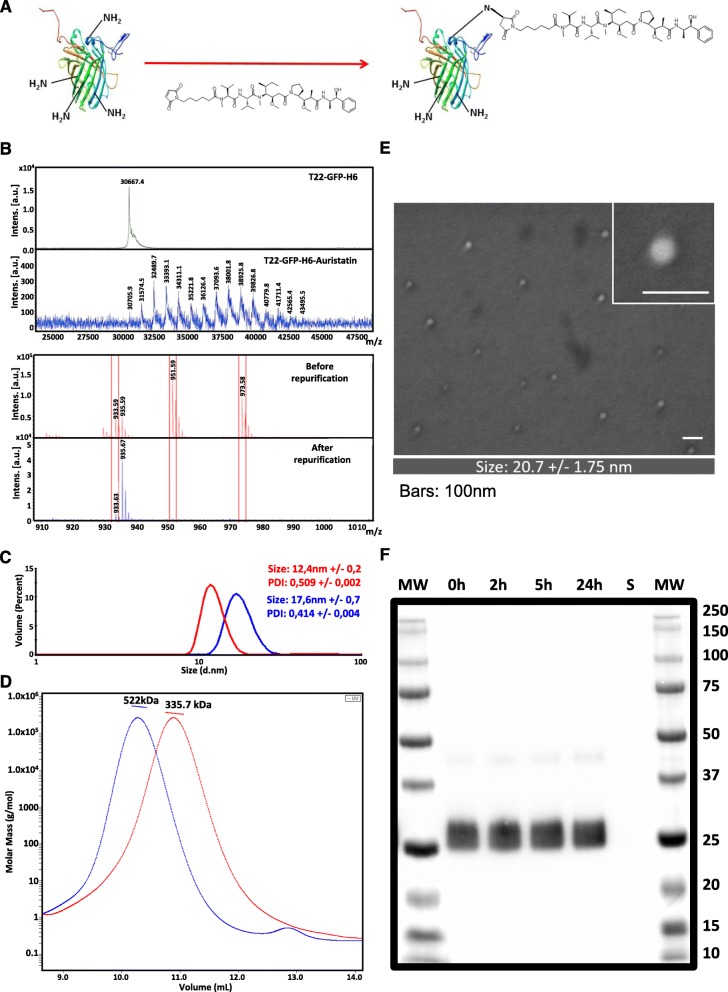
Fig. 1.
T22-GFP-H6-Auristatin nanoconjugates characterization and stability. a T22-GFP-H6 nanoparticle conjugation with maleimide functionalized Monomethyl Auristatin E (MMAE). b MALDI-TOF mass spectrometry of wild-type T22-GFP-H6 nanoparticles and T22-GFP-H6-Auristatin nanoconjugates (top) and free MMAE before and after nanoconjugates re-purification (bottom). Each peak corresponds to the covalent addition (+ 911 Da) of a single MMAE molecule (top). Red boxes indicate free Auristatin mass spectrometry spectra (bottom). c Hydrodynamic volume size distribution of wild-type T22-GFP-H6 nanoparticles (red) and T22-GFP-H6-Auristatin nanoconjugates (blue) determined by dynamic light scattering. Samples were analyzed in triplicate and data represented as mean +/− SE. PDI indicates polydispersion index. d Average molar mass distribution of wild-type T22-GFP-H6 nanoparticles (red) and T22-GFP-H6-Auristatin nanoconjugates (blue) determined by size exclusion chromatography coupled to multi-angle light scattering (SEC-MALS). e Representative electron microscopy (FESEM) images of T22-GFP-H6-Auristatin nanoconjugates presented in two magnifications (see inset). Scale bars indicate 100 nm. In the bottom, the quantitative average size of nanoconstructs determined by image analysis and shown as mean ± SE. f Proteolytic stability of T22-GFP-H6-Auristatin nanoconjugates in human serum at different incubation times up to 24 h analyzed by western blot immunodetection with a monoclonal anti-His antibody. “S” indicates human serum control. MW, molecular weight; SE, standard error