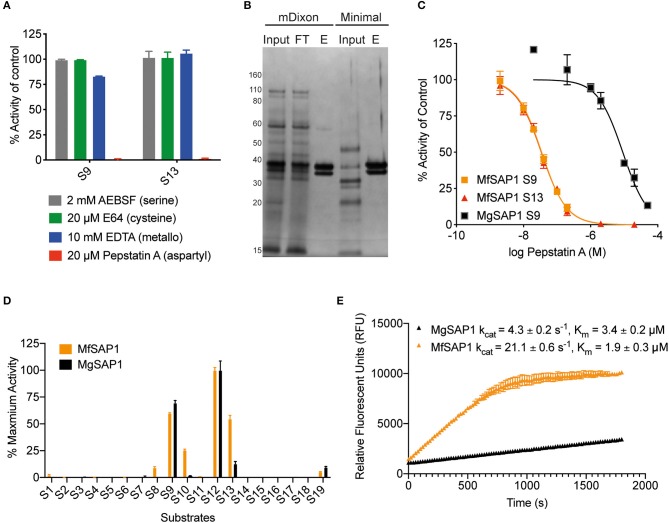
Figure 2.
Identification of the major aspartyl protease MfSAP1 in M. furfur and comparison with its M. globosa homolog MgSAP1. (A) M. furfur extracellular media was treated with each protease inhibitor and the remaining protease activity was assessed using S9 and S13. (B) Silver stain of the extracellular media (input), the enriched elute (E) and the flow-through (FT) from the pepstatin A-agarose affinity purification of the secreted proteases in M. furfur culture grown in mDixon and minimal media. (C) Inhibition curves of the enriched proteases MfSAP1 and MgSAP1 against the aspartyl protease inhibitor pepstatin A. (D) Comparison of the substrate cleavage preferences of MfSAP1 and MgSAP1 for the quenched substrates S1-S19. Protease activities were normalized to the maximum activity of the panel (S12) in each enzyme. (E) Kinetic parameters of enriched MfSAP1 and MgSAP1 for cleavage of substrate S12. A representative plot at 20 μM of S12 is shown for both enzymes. The Michaelis-Menten plot for MfSAP1 is included in Supplementary Figure 1. The data for MgSAP1 is previously published in Li et al. (2018). Error bars represent standard deviation for n = 3.