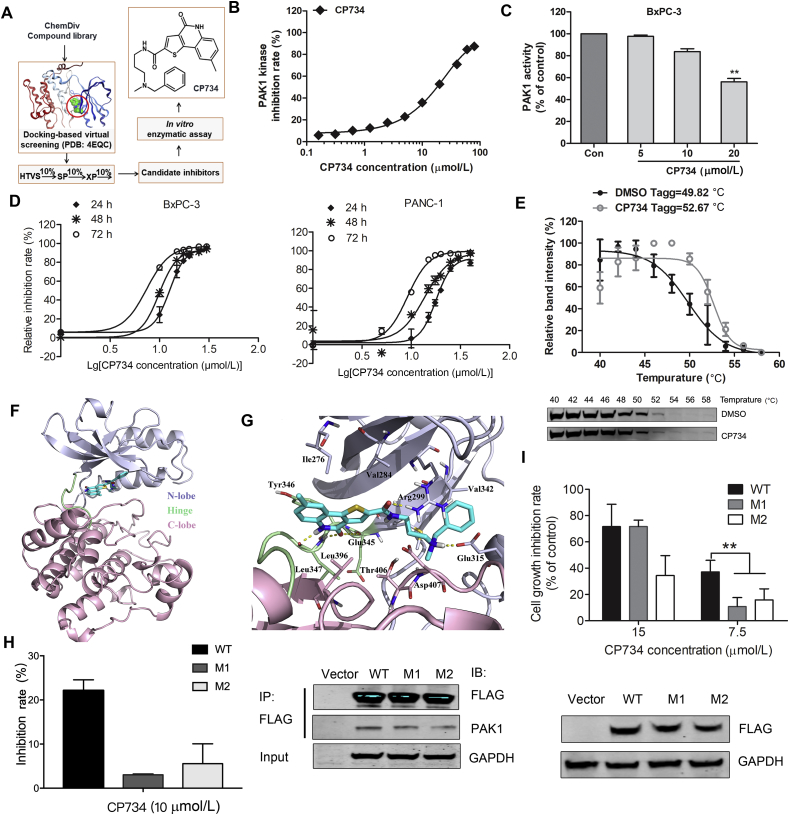
Figure 3.
Identification of CP734 as a potent PAK1-targeted inhibitor. (A) Schematic representation of hit discovery strategy. HTVS: high-throughput virtual screening mode; SP: standard-precision mode; XP: extra-precision mode. (B) In vitro inhibition profiles for CP734 against PAK1 in kinase reaction. (C) Inhibitory capacity of CP734 against intracellular PAK1 activity in BxPC-3 cells. (D) Cytotoxicity of CP734 towards BxPC-3 and PANC-1 cells. (E) The cellular thermal curve shift of PAK1 treated with CP734 (20 μmol/L). (F) Overall structure of PAK1 with CP734 bound in the ATP-binding site. The N-terminal lobe is shown in light gray, the hinge region is shown in light green, and the C-terminal lobe is shown in pink. (G) View of the PAK1 active site in complex with CP734. Residues that form the binding pocket are labeled. (H) Mutants V342F-PAK1 (M1) and V342K-PAK1 (M2) decreased the in vitro inhibition effect of CP734 compared with that of wide-type PAK1 (WT). Immunoprecipitation of the FLAG-tagged WT, M1 and M2 in HEK293 cells, and blotted with anti-FLAG and anti-PAK1 antibodies. (I) M1 and M2 attenuated the cell-growth inhibition activity of CP734 compared with that of WT. Overexpressed WT, M1 and M2 in BxPC-3 cells was detected by Western blot. All the data are represented as mean ± SD, n = 3. **P < 0.01, significantly different.