Figure 8.
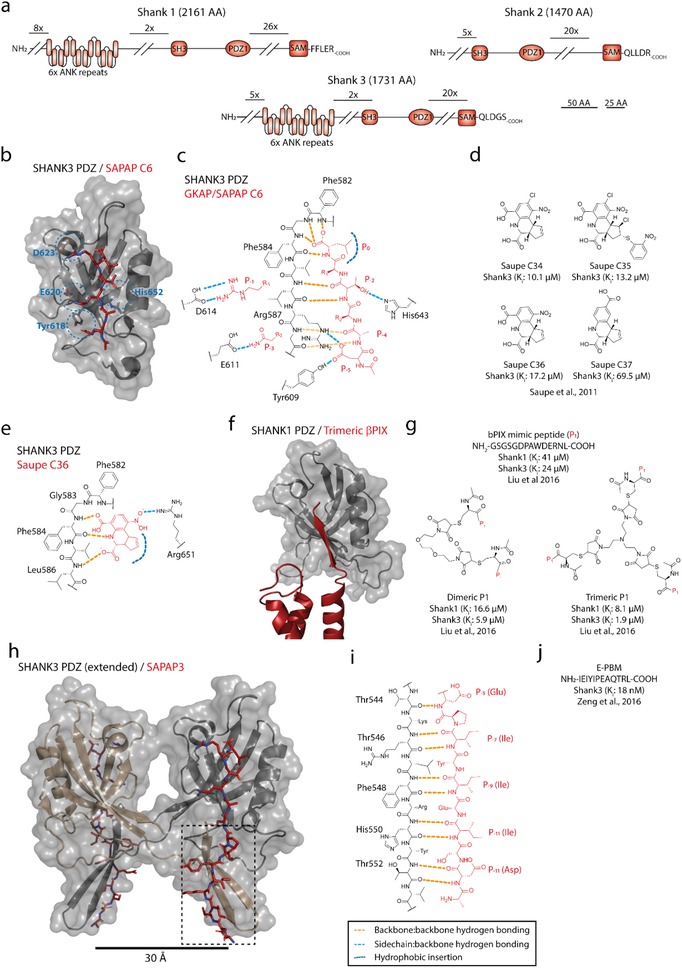
a) Domain organization of Shank family proteins (Uniprot: Q9Y566, Q9UPX8, Q9BYB0). b,c) Canonical insertion of a type I ligand (ac‐EANTRL‐COOH, red) into the PDZ domain of Shank3, with canonical hydrogen binding network, hydrophobic insertion of P0 (Leu) and P−2 (Thr) coordination with His643; furthermore, the interactions are supported by side chain interactions between P−1(Arg)/Asp614 and P−3(Gln)/Glu611. d). Small‐molecule inhibitors targeting the PDZ domain of Shank3. e) Hydrogen binding network of C36 (red) binding to the PDZ domain of Shank3, mimicking the backbone hydrogen bonding network of the P0 and P‐1 residues from canonical ligands, and hydrophobic insertion of cyclopentene moiety; the binding is further stabilized by the interaction between the nitro group (─NO2) and Arg651. f) Binding of trimeric β‐PIX (red) to the PDZ domain of Shank3 shows that steric hindrance reduces the expected avidity effect expected from close proximity.334 g) Monomeric, dimeric, and trimeric peptide analogues mimicking βPIX binding to the PDZ domain of Shank1 and Shank3. h) Structure of SAPAP C15 (NH2‐ADSIEIYIPEAQTRL‐COOH, red) binding to an extended variant of the PDZ domain of Shank3 shows ligand‐induced PDZ‐PDZ’ dimerization (PDB: 5IZU). i) Hydrogen binding network of β‐strand/β‐strand coordination between SAPAP3 P−5 and P−14 (red) and the Shank3 PDZ domain extension accounts immense affinity enhancement and ligand specificity. j) Primary sequence of the SAPAP3 C15.
