Figure 5.
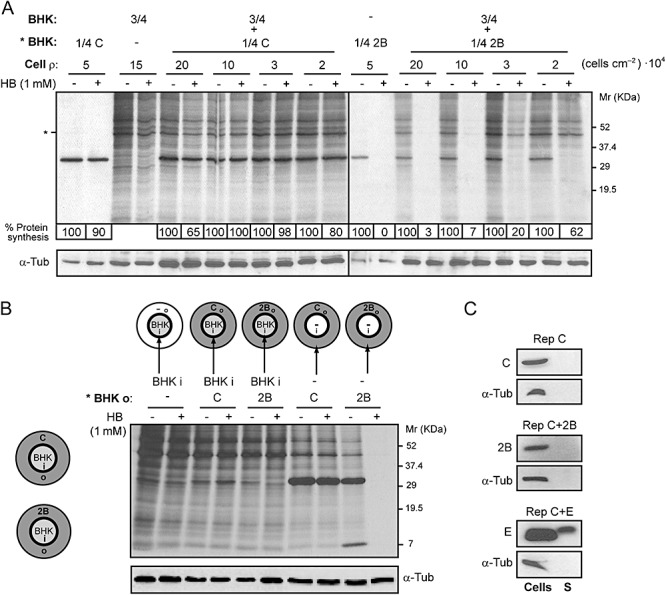
Permeabilization of neighbouring cells to HB is dependent on cell–cell contact. A. A fixed proportion of transfected (with replicons encoding C+2B or C alone; * BHK) and mock cells (*BHK : BHK, 1:3; total number of cells = 4 × 105; cell ρ at maximum confluence = 20 × 104 cells cm−2) was seeded on plates with different growth areas (2, 3.8, 11.8 and 19.5 cm2). At 8 h post electroporation (hpe), permeabilization of mock cells was assayed by the inhibition of translation as a result of HB entry induced by 2B protein. Protein synthesis in mock cells was quantified by densitometry of bands corresponding to actin (*). Numbers below the gel indicate the percentage of protein synthesis in HB‐treated cells compared with untreated cells. As a control for protein load, α‐tubulin was detected by Western blotting (lower panel). B. Permeabilization of BHK cells is not induced when cell contact with 2B‐expressing cells is not established. Schematic drawings of culture plates (growth area = 11.8 cm2) containing a central ring that divides the plate into an independent inner (i) chamber (growth area ≈ 2 cm2) and an outer (o) concentric region (left). Cells transfected with replicons encoding C+2B or C alone were seeded on the outer region of the plate (grey) and mock cells were independently seeded into the central chamber (white). Once cells were settled, the central ring was removed in such a way that all cells shared the same culture medium. At 8 hpe, cells were metabolically labelled for 40 min in the absence (−) or presence (+) of 1 mM HB. Transfected and mock cells were independently collected in loading buffer by first replacing the central ring the culture plate. Mock cells (from the central chamber) and transfected cells (outer region) expressing C+2B or C (negative control) were loaded separately, as indicated in the figure (right panel), and processed by SDS‐PAGE. It can be observed that only cells that express 2B protein are permeable to HB. As a protein loading control, α‐tubulin was detected by Western blotting (lower panel). C. 2B protein is not released into the culture medium. Cells expressing C alone, 2B or E protein from MHV‐A59 (positive control) and their respective culture media were collected separately at 8 hpe. Cells were resuspended in loading buffer while culture media were centrifuged and proteins from supernatants (S) were precipitated using trichloroacetic acid (see Experimental procedures) and resuspended in loading buffer. The presence of viral proteins in cells and supernatants was analysed by Western blotting using rabbit polyclonal antibodies directed against C, 2B and E. The absence of cellular proteins in supernatant was confirmed by Western blotting using monoclonal antibodies specific for α‐tubulin.
