Figure 4.
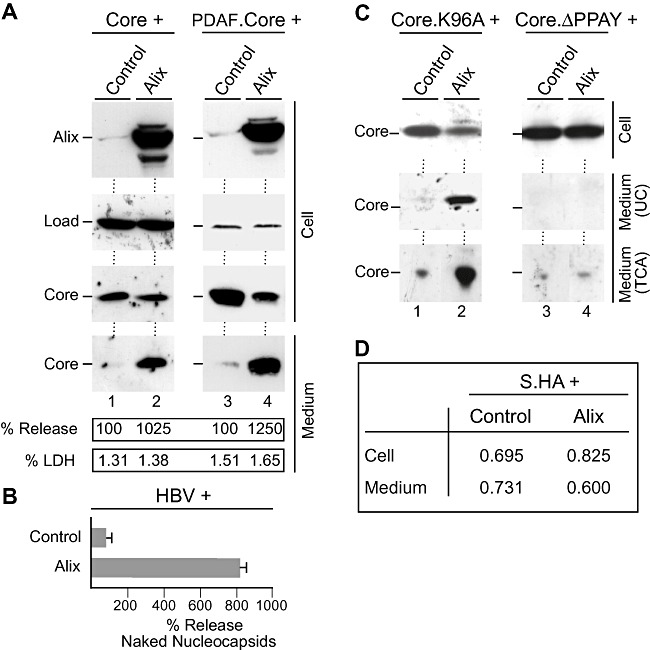
Excess Alix enhances HBV naked (nucleo)capsid release. A. Core (lanes 1 and 2) or DPAF‐tagged core (lanes 3 and 4) was co‐transfected with control DNA or HA‐tagged Alix at a 1:3 DNA ratio respectively. Cell extracts were prepared with SDS and analysed by HA‐specific immunoblotting to monitor expression of Alix. For detection of core, the core antiserum (K46) (lanes 1 and 2) or the DPAF antibody (lanes 3 and 4) was used. In either case, non‐specifically stained bands served as a control for gel loading (Load). Capsids harvested from the culture media were analysed in the same manner. The experiments were repeated three times, and capsids released into the supernatants were quantified and demonstrated in per cent amount relative to control cells. To probe for cell lysis, supernatants were assayed for LDH activity. B. The pHBV replicon was co‐transfected with Alix or control plasmid DNA. Cell supernatants were immunoprecipitated with the capsid‐specific antiserum (K45) prior to PCR measurement of the viral DNA. Mean PCR results are demonstrated in per cent amount relative to control‐transfected cells. C. The core mutants Core.K96A (lanes 1 and 2) and CoreΔPPAY (lanes 3 and 4) were subjected to the co‐transfection assay exactly as in (A). Cell lysates and media concentrated by either ultracentrifugation (UC) or TCA precipitation (TCA) were immunoblotted with the core antiserum (K46). D. An HA‐tagged version of the HBV small envelope protein (S.HA) was transfected into HuH‐7 cells together with Alix or a control construct at a 1:3 ratio respectively. Amounts of S were examined by ELISA and are expressed as mean units of optical density at 492 nm (n = 3).
