Figure 1.
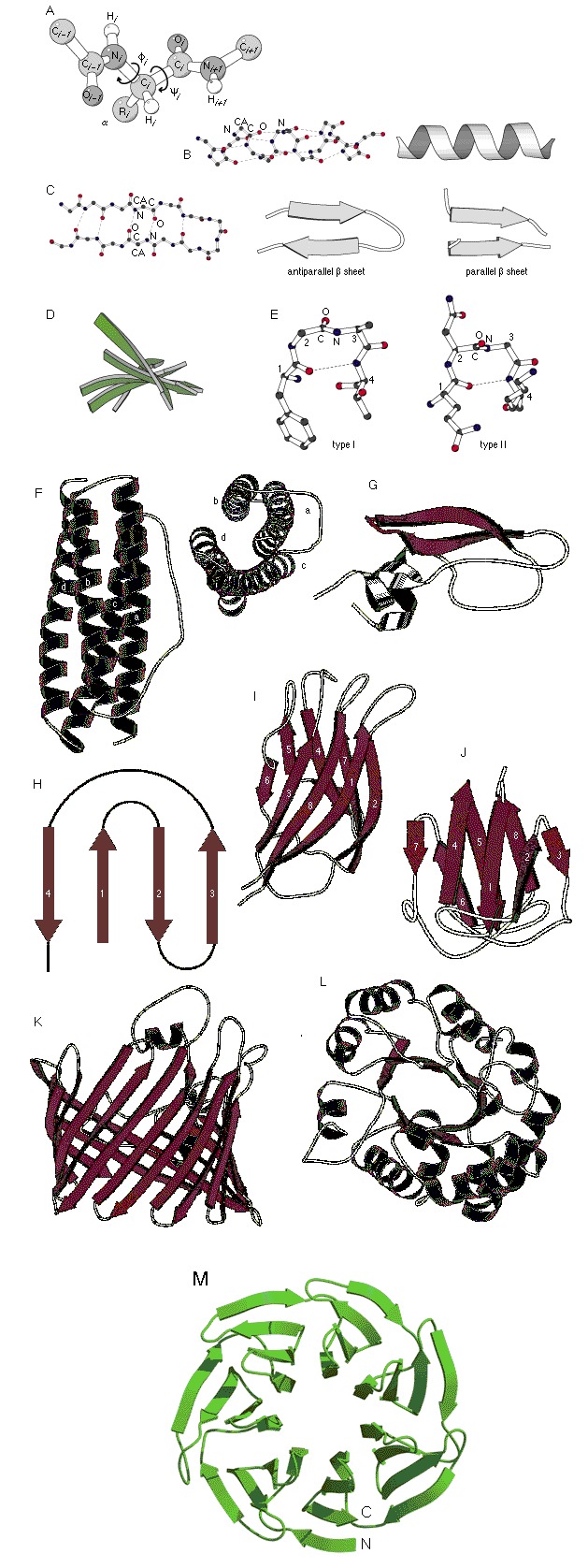
(A) Drawing of an l‐polypeptide chain using a ball‐and‐stick model to illustrate torsion angles φ and ψ for residue i. Torsion angle φ defines the angle between the planes specified by atoms Ci‐1–Ni–Ci α and Ni–Ci α–Ci, respectively. Torsion angle ψ defines the angle between the plane specified by atoms Ni–Ci α–Ci and Ci α–Ci–Ni+1 respectively. Also shown are both ball‐and‐stick and ribbon representations of an (B) α‐helix and (C) β‐sheet. The latter is shown in both anti‐ and parallel orientations. (D) Illustration of the characteristic right‐handed twist of a β‐sheet as observed in flavodoxin (PDB entry 1flv). (E) Types I and II tight turns. Examples of commonly observed secondary structure assemblies: (F) four‐helix bundle (top and side view; PDB entry 1bcf), (G) β‐hairpin structure (PDB entry 1bpi), (H) β‐sheet with Greek key topology (topology diagram), (I) jelly‐roll motif (PDB entry 1pgs); (J) β‐sandwich (PDB entry 4gcr), (K) 16‐stranded β‐barrel (PDB entry 2por), (L) α/β‐barrel (PDB entry 1btm), and (M) seven‐bladed β‐propeller (PDB entry 1got).
