Figure 4.
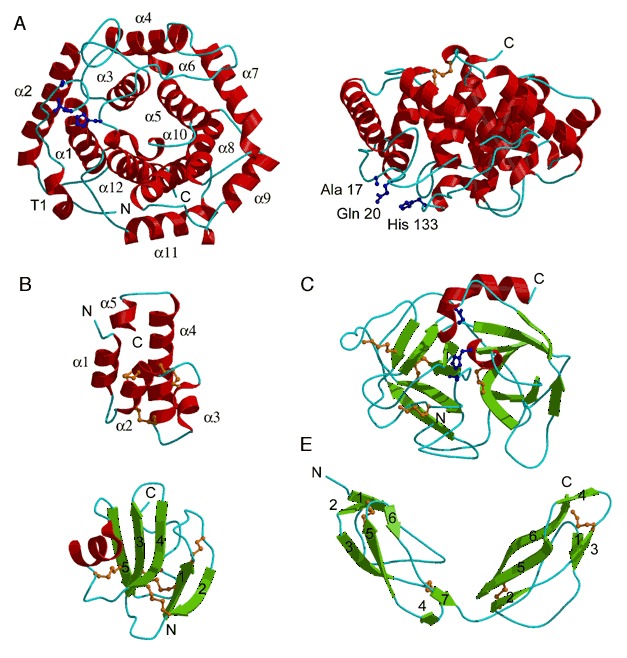
Protein folds in the complement system. (A) C3d (PDB entry 1c3d; residues 996 to 1287). Left side shows the view down the barrel axis; the right side shows the side view of the barrel. The α helices are numbered 1 to 12 and the N‐terminal 310 helix is labeled T1. The residues critical for covalent attachment to the pathogen surface, His 133, Gln 20, and Ala 17 (Cys residue in the wild type protein), are represented by a ball‐and‐stick model. (B) C5a (PDB entry 1kjs).The α helices are numbered from 1 to 5 (C) Complement factor D serine protease (PDB entry 1dsu). Catalytic residues His 57, Asp 102, and Ser 195 are represented by a ball‐and‐stick model. (D) Complement regulatory protein CD59 (PDB entry 1cdq; residues 1 to 70). The β strands are numbered according to their order in the protein sequence. (E) CCP modules 15 and 16 from complement factor H (PDB entry 1hfh). The β strands for each separate CCP module are numbered according to their order in the protein sequence.
