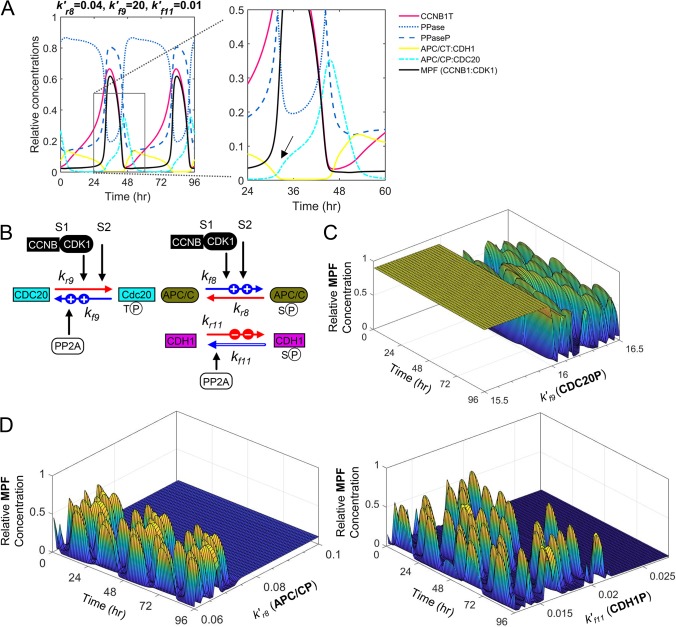
Fig 8. Varying rates of site-specific phosphorylation and dephosphorylation drive mitotic exit.
(A) Simulation of PPase phosphatase (i.e. PP2A) and MPF kinase (CCNB1:CDK1) in regulating activation of APC/CP:CDC20 and APC/CT:CDH1 over the indicated time periods with sequential loses in CCNB1T, MPF, PPaseP, APC/CP:CDC20 and APC/CT:CDH1. Boxed area is magnified, and arrow identifies the approximate onset of APC/C:CDC20 activation. (P, phosphorylated protein; T, total protein) (B) MPF and PP2A substrate preferences for S (serine) and T (threonine), respectively, drive mitotic progression. This includes MPF-dependent slower phosphorylation of CDC20 (k′r9) and faster phosphorylation of APC/C (k′f8) and CDH1 (k′r11), and faster PP2A-dependent dephosphorylation of CDC20P (k′f9) and slower dephosphorylation of APC/CP (k′r8) and CDH1P (k′f11). (C) Simulated increases in PP2A-dependent dephosphorylation of CDC20P () result in increased oscillations of active MPF kinase occurring when is high. (D) Simulated increases in PP2A-dependent dephosphorylation of APC/CP () and CDH1P () result in loss of oscillations of active MPF kinase when and are high, respectively.