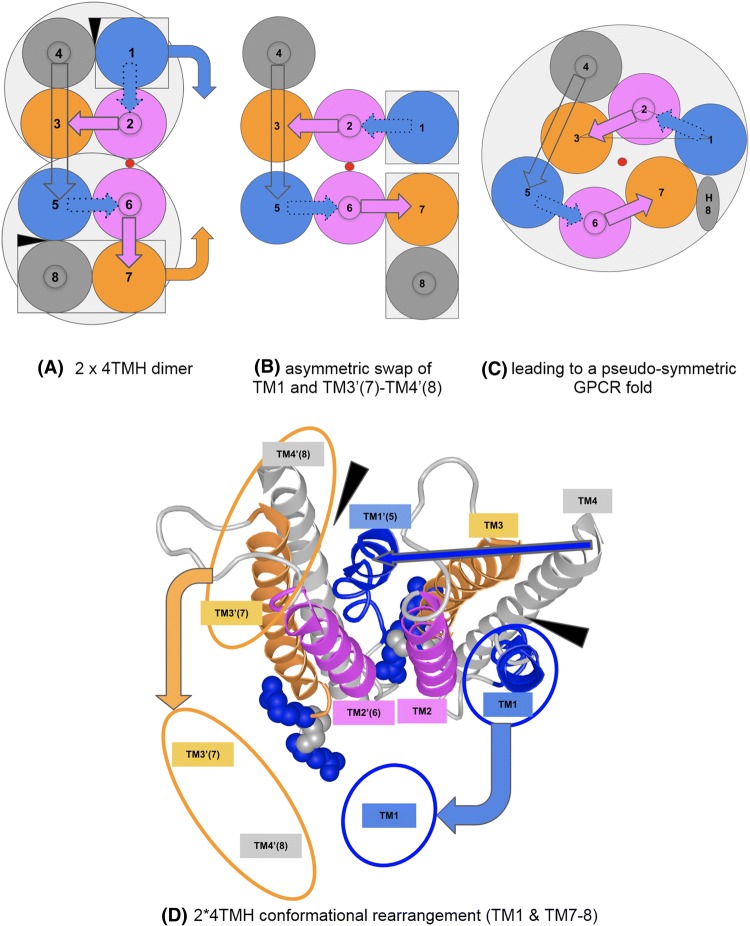
Fig. 5.
GPCR fold formation fusing two 4TMH protodomains with conformational change: Concerted asymmetric subdomain swap TM3–TM4 vs. TM1. A rearrangement scenario involving a rigid swap at the interface TM1–TM4 of both 4TMH domains forming a dimer (a) under a TM4(1)–TM1(2) linker constraint. This would involve a conformational change and transition from a dimer of 4TM-protein binding G-proteins to a GPCR configuration through an asymmetric swap (b) of one helix TM1 on one monomer vs. a two helices TM3′–TM4′ (TM7–TM8) on the second to obtain a symmetric GPCR arrangement (c) of TM1–2–3 vs TM5–6–7. d This scenario overlaid on a 3D structure of the nicotinic acetylcholine α4β2 Receptor [pdbid: 6CNK (Walsh et al. 2018)]