Table 2.
Q-tube [4 + 2] cyclocondensation reactions of 4-thiazolidinone 4a-c and arylhydrazonal 5.
| Entry | Reactants | Ar1 | Ar2 | X | Product | Yield (%)a |
| 1 | 4a + 5a | C6H4 | C6H5 | 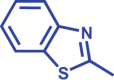 |
 |
98 |
| 2 | 4a + 5b | C6H4 | 4-Cl-C6H4 |  |
 |
99 |
| 3 | 4a + 5c | C6H5 | 4-NO2-C6H4 | 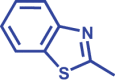 |
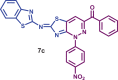 |
94 |
| 4 | 4a+5d | C6H5 | 4-MeO-C6H4 | 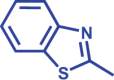 |
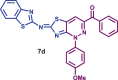 |
95 |
| 5 | 4a + 5e | 4-Cl-C6H4 | 4-Cl-C6H4 |  |
 |
96 |
| 6 | 4a + 5 f | 4-Cl-C6H4 | 2-Cl-5-NO2-Ph |  |
 |
98 |
| 7 | 4a + 5 g | 4-Br-C6H4 | 2-Cl-5-NO2-Ph |  |
 |
97 |
| 8 | 4a + 5 h | 4-Br-C6H4 | 2,4-diF-C6H3 |  |
 |
94 |
| 9 | 4a + 5i | 4-F-C6H4 | 4-Me-C6H4 |  |
 |
92 |
| 10 | 4a + 5j | C10H7 | 4-Cl-C6H4 |  |
 |
96 |
| 11 | 4a + 5k | C10H7 | 4-NO2-C6H4 |  |
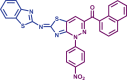 |
95 |
| 12 | 4a + 5 l | C10H7 | 4-NO2-C6H4 |  |
 |
93 |
| 13 | 4a + 5 m | C4H3S | C6H5 |  |
 |
92 |
| 14 | 4a + 5n | C4H3S | 4-Cl-C6H4 | 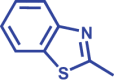 |
 |
93 |
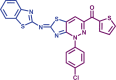
| 15 | 4a + 5o | C4H3O | 2-Cl-5-NO2-Ph | 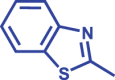 |
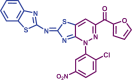 |
90 |
| 16 | 4a + 5p | N-Me-Ind | 4-NO2-C6H4 |  |
 |
91 |
| 17 | 4b + 5a | C6H5 | C6H5 |  |
 |
91 |
| 18 | 4b + 5e | 4-Cl-C6H4 | 4-Cl-C6H4 | 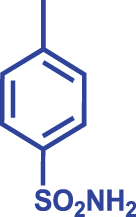 |
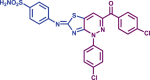 |
94 |
| 18 | 4b + 5c | C6H5 | 4-NO2-C6H4 |  |
 |
92 |
| 19 | 4c + 5a | C6H5 | C6H5 |  |
 |
88 |
| 20 | 4c + 5b | C6H5 | 4-Cl-C6H4 |  |
 |
89 |
aReaction conditions: a mixture 4-thiazolidinone 4a-c (5 mmol), arylhydrazonal 5 (5 mmol), anhydrous sodium acetate (10 mmol) and glacial acetic acid (10 mL) was charged in the glass tube of the Q-tube reactor and heated in an oil bath at 170 °C for 25 min.
