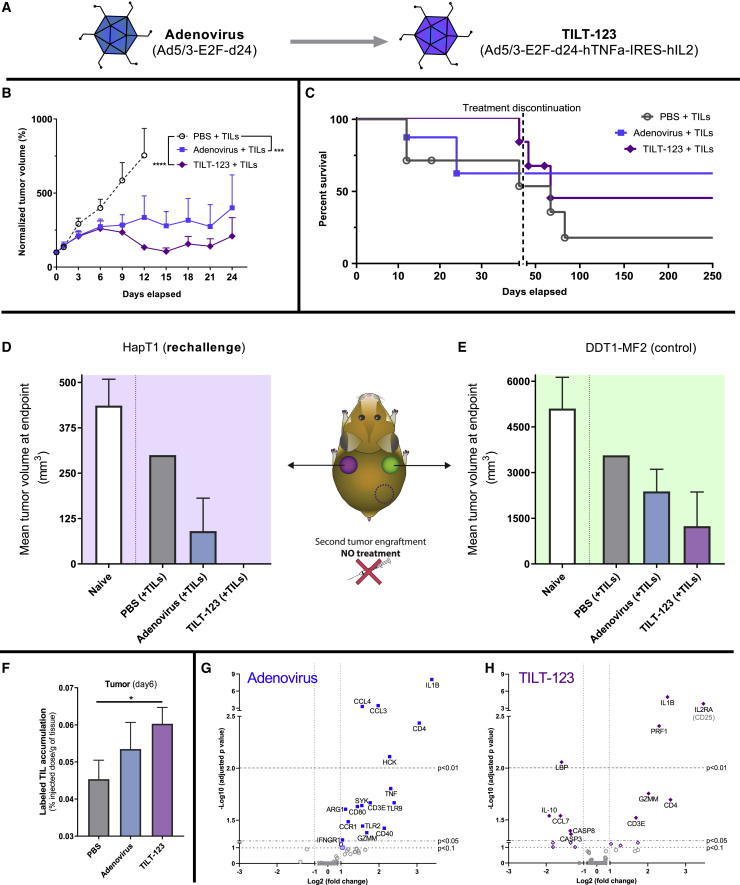
Figure 5.
Antitumor and Immunological Effects of Arming an Adenovirus with Immunostimulatory Cytokines
(A) Summary of unarmed and armed virus constructs. (B) Grouped-normalized tumor volume values for the different groups (n = 6–9 per group) after receiving TIL therapy (intraperitoneally) and virotherapy or PBS (intratumorally). Treatment schedule is presented in Figure 1A. (C) Overall survival data. Gray dashed line marks discontinuation of the treatments. (D) Mean tumor volume at day 19 after HapT1 rechallenge in complete responders from HapT1 tumors. (E) Mean tumor volume at day 19 after DDT1-MF2 challenge in complete responders from HapT1 tumors (naive, n = 3; PBS + TILs, n = 1; adenovirus + TILs, n = 5; TILT-123 + TILs, n = 2). (F) 13 Syrian hamsters carrying subcutaneous HapT1 tumors were randomized into groups and treated with 111indium-labeled TILs intraperitoneally and with PBS (n = 4), unarmed adenovirus (n = 5), or armed adenovirus (n = 4). At day 6 of the experiment, tumors were harvested for ex vivo111indium measurement by a gamma counter (∗p < 0.05). Comparisons were made between the RNA expression profiles from each of the two adenovirus-treated groups versus the PBS-treated group. Animals carrying these tumors were treated as described in Figure 3A. Viral treatment used in the group compared to PBS-treated animals: (G) adenovirus and (H) TILT-123. The plots indicate the names of those genes for which there is a statistically significant difference (adjusted p value < 0.05) and an expression change over double or below half to those in the reference group (−1 > log2 fold change > 1). All error bars are SEM.