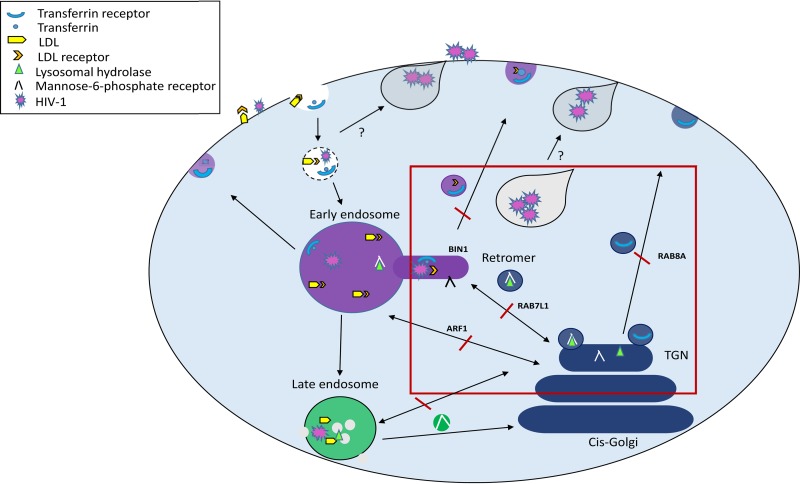
FIG 8.
Model for the roles of ARF1, BIN1, RAB7L1, and RAB8A in the endocytic pathway and vesicle formation in MDDCs. Molecules are internalized from the cell surface via endocytic vesicles that fuse with each other or existing endocytic vesicles to form early endosomes. The budding of vesicles containing cargo from early endosomes to the plasma membrane and trans-Golgi network (TGN) requires the activity of BIN1. TGN vesicles bud from the TGN surface and either fuse with each other or endocytic compartments. The TGN is responsible for sorting receptors from degradative compartments and delivers newly synthesized lysosomal enzymes in the form of lysosomal hydrolase via the mannose-6-phosphate receptor. Both transferrin and LDL are taken into the cell via clathrin-receptor-mediated endocytosis. Transferrin and its receptor are recycled from early endosomes back to the plasma membrane. LDL is trafficked directly to lysosomes prior to release into the cytoplasm. The dynamic retrograde transport of vesicles between the TGN and endocytic compartment and the plasma membrane via the retromer and other trafficking pathways depends on the activity of ARF1, RAB7L1, and RAB8A. HIV-1 trans-infection between MDDCs and CD4+ T cells requires a homeostatic balance of the endocytic pathway. By blocking trafficking of molecules between early endosomes and the TGN and onward polarized transport of cargo to the plasma membrane, HIV-1 trans-infection is inhibited. Depletion of targeted proteins results in the accumulation of HIV-1 in intracellular vesicles that are unable to traffic to the virological synapse.